Abstract
Background?Bronchiectasis is a chronic inflammatory disease with no targeted therapy. HSK31858 is a novel inhibitor of dipeptidylpeptidase-1(DPP-1) for the treatment of bronchiectasis.
Objectives: We aimed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple oral doses of HSK31858 of administered orally.
Methods: This was a randomized, double-blind,placebo-controlled, dose-escalation study. In Part A, healthy subjects were randomized to receive single ascending oral doses of HSK31858(15,40,60 and 80mg) or placebo. In Part B, healthy subjects were randomized to receive multiple(qd,28-day) ascending oral doses of HSK31858(10,20 and 40mg) or placebo. Whole blood NE activity was measured on specified days.
Results: No adverse trends were observed in laboratory findings, 12-lead ECGs. There was no signal of increased infection risk. HSK31858 was absorbed rapidly, with no significant accumulation of Cmax, while AUC accumulation was more pronounced. There was dose-dependent inhibitory effect in whole blood NE activity, with maximum inhibition of 13.6%, 44.1%,and 76.4% in the 10mg,20mg,and 40mg groups,repectively.
Conclusions: HSK31858 exhibited a favorable safety and tolerability profile, and dose-dependently inhibited whole blood NE activity.
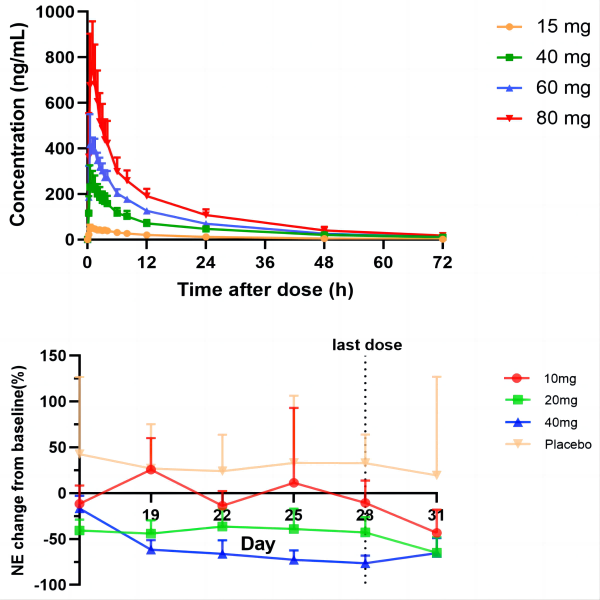
Figure1. PK profiles of single dose of HSK31858 and mean change in whole blood NE activity