Abstract
Introduction or background: Multiple tools are available to measure persistent breathlessness, but which to use is unclear.
Aims and objectives:To compare concurrent self-ratings of four measures of breathlessness over one week from a randomised clinical trial of sustained-release (SR) morphine for persistent breathlessness.
Methods: Participants with optimally-treated persistent breathlessness and a clinician-rated modified Medical Research Council (mMRC) score?2 were randomised to once-daily SR morphine 20mg or placebo for one week. Breathlessness measures were unpleasantness now and intensity of worst, best and average breathlessness (previous 24 hours), measured on a 0-100mm visual analogue scale. On each scale, change from baseline to days 5-7 was analysed using logistic regression, for all participants and subgroups by mMRC and diagnosis (chronic obstructive pulmonary disease vs. others), alone and combined.
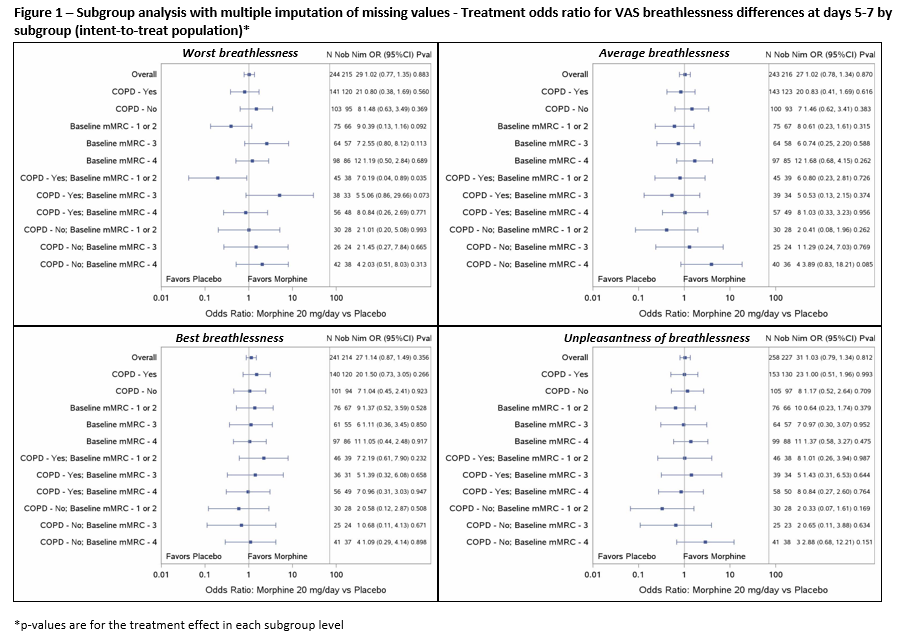
Results: 264 participants were included (mean age 74.0±[SD]9.2 years; 63.6% male). Distribution of scores and precision (95% confidence intervals) were similar between scales (Figure 1). For worst breathlessness, there appears to be more variability in the point estimates for the COPD/mMRC?2 and COPD/mMRC 3 subgroups (Figure 1).
Conclusion: There is no indication to prioritise one measure of breathlessness over another. People?s subjective experience of change based on the cause and severity of breathlessness should be explored.