Abstract
Asthma is a common and chronic respiratory disease. Some asthmatic patients still experience severe symptoms despite appropriate therapies, substantiating a need for novel and effective treatments. Eosinophils are immune cells derived from hematopoietic stem cells (HSCs), which are critical in asthmatic airway pathogenesis. However, their regulatory functions have not been well elucidated.
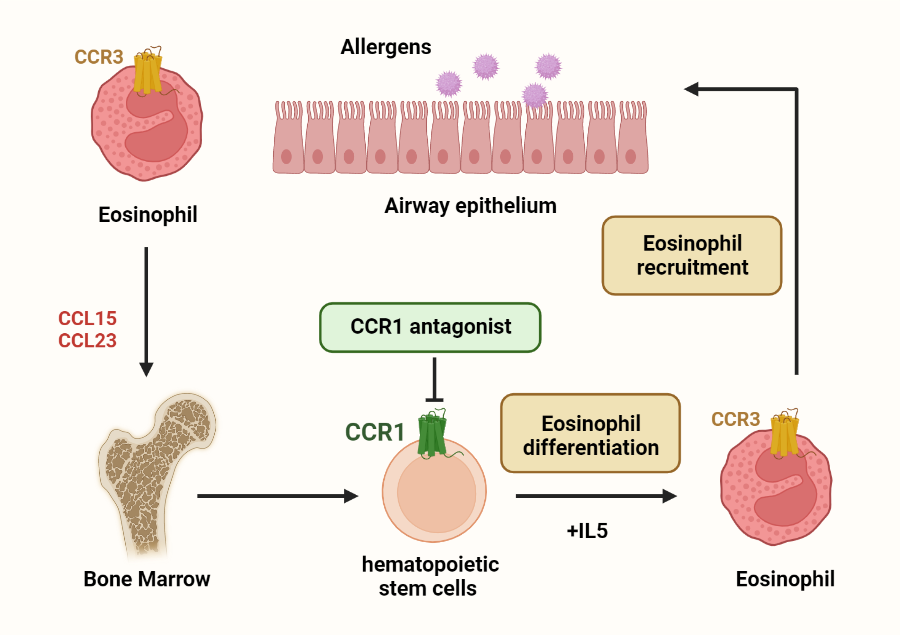
Our previous studies indicated that eosinophils disrupted HSC homeostasis by impairing HSC quiescence and reconstitution ability. By using mass spectrometry and mice models, we identified chemokine CCL6, which was derived from eosinophils, to impair HSC homeostasis. The human orthologs of CCL6, chemokines CCL15 and CCL23, were observed increased in asthmatic patients. These chemokines were endogenous agonists of receptor CCR1. Eosinophil differentiation and airway inflammation were remarkably decreased by CCR1 antagonist BX471, supporting that blocking CCR1 may be an effective way to treat eosinophilic inflammation in asthma.
No therapeutic molecules targeting CCR1 have been approved. By using single-particle cryo-electron microscopy, we recently solved the structures of CCR1 in complex with CCL15 and its antagonist BX471. Together with functional results, our studies revealed the structural basis of ligand recognition and receptor activation of CCR1, as well as provided multiple templates for rational design of therapeutics targeting CC chemokine receptors.