Abstract
Background: Leukotriene receptor antagonist including montelukast, may have neuropsychiatric adverse effects, but findings have been conflicting, also in paediatric patients.
Objective: To assess if montelukast exposure in children with asthma is associated with onset of neuropsychiatric adverse events using data from the Danish nationwide health registers.
Methods: Children aged 5-17 years with either ?1 prescription redemption of inhaled corticosteroids or montelukast, or with at least one hospital contact with asthma between January 1, 2010 and December 31, 2018 were included.
Outcomes were: use of neuropsychiatric medicine (Outcome 1), and hospital contacts with a neuropsychiatric diagnosis (Outcome 2). All outcomes were assessed at 90 days of exposure to montelukast. Children who used neuropsychiatric medicine at baseline were excluded in assessment of Outcome 1.
Results: We identified 193,994 children with asthma. No association was found between initiation of montelukast and Outcome 1 or Outcome 2, respectively (HR (95% CI) = 1.08 (0.97-1.18), p=0.18, and HR (95% CI) = 0.92 (0.77-1.10), p=0.36).
Conclusion: Initiating montelukast in children with asthma was not associated with the onset of neuropsychiatric events.
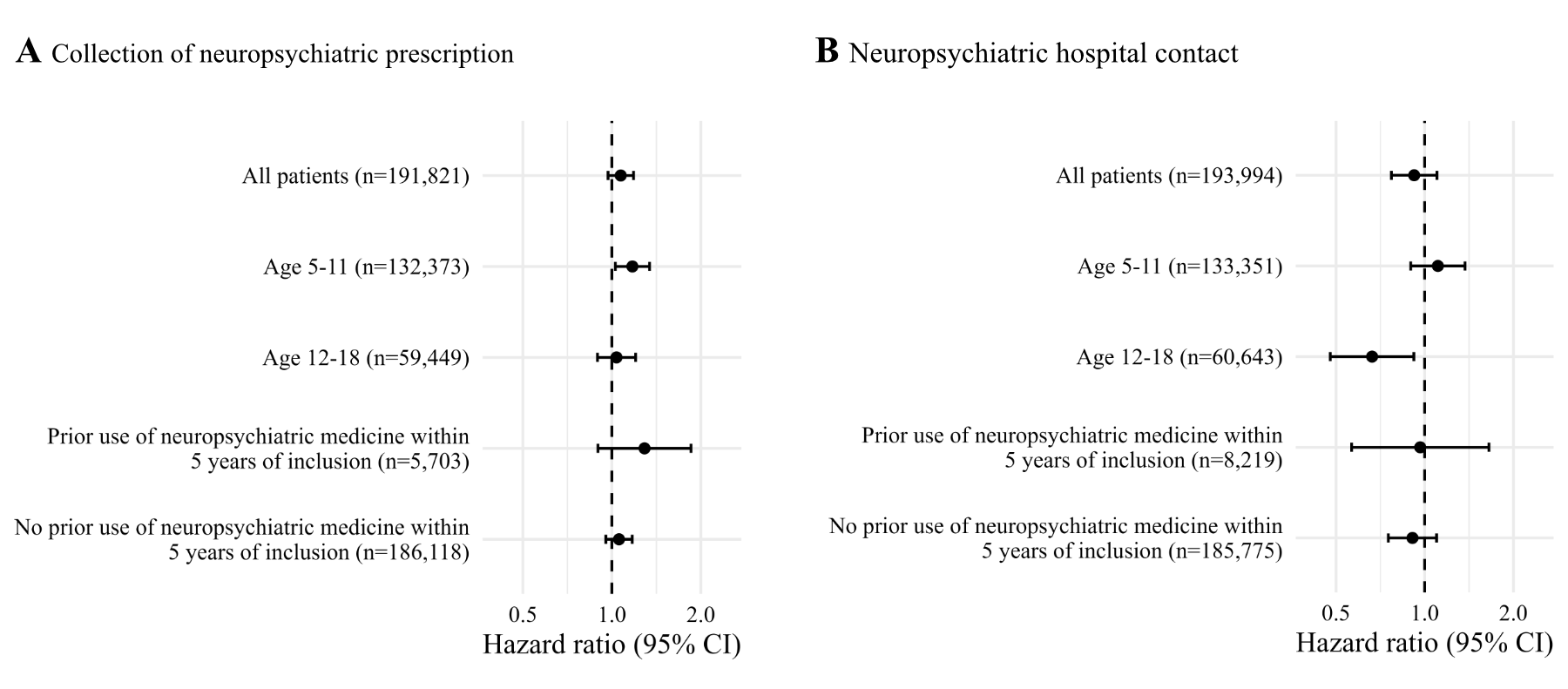
Figure 1: Risk of neuropsychiatric conditions associated with montelukast initiation assessed as use of neuropsychiatric medicine and hospital contact with a neuropsychiatric diagnose.