Abstract
Background
In the Phase 2 WILLOW study (NCT03218917), brensocatib, an investigational dipeptidyl peptidase-1 (DPP-1) inhibitor, prolonged time to exacerbation in non?cystic fibrosis bronchiectasis (NCFBE) patients (pts). Cystic fibrosis (CF) pts share many pathophysiologic features with NCFBE pts.
Objective
To assess pharmacokinetics (PK) and safety of brensocatib in CF pts and compare with heathy subjects and NCFBE pts.
Methods
This was a Phase 2, single-blind, placebo-controlled study (NCT05090904) in pts with/without exposure to CF transmembrane conductance regulator (CFTR) modulators who received 10, 25, or 40 mg brensocatib or placebo orally once daily for 4 weeks.
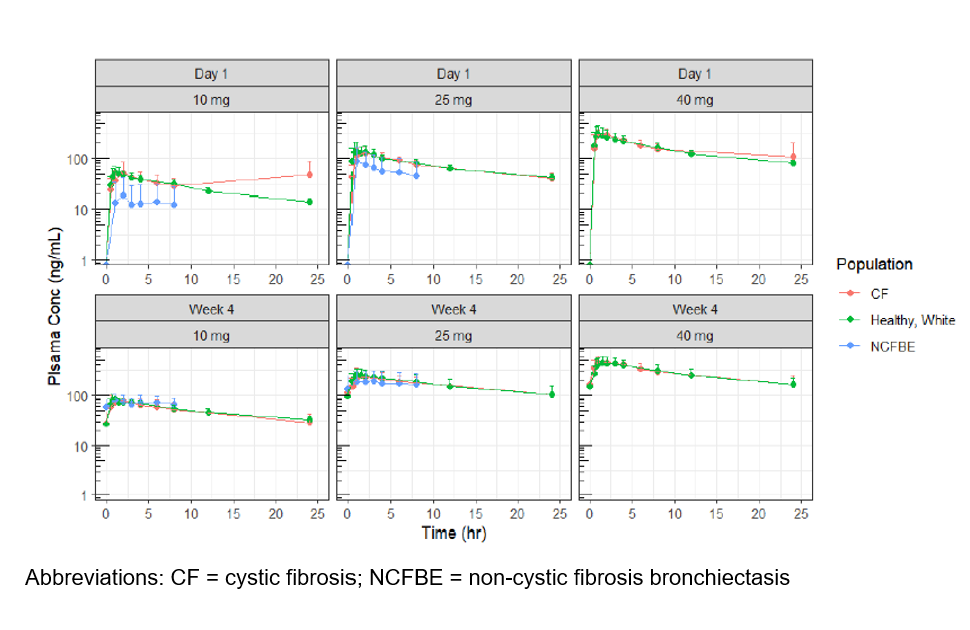
Results
Twenty-nine pts (mean age, 37.9 y; 62.1% men; mean percent predicted FEV1, 64.9; CFTR modulator use, n=21) were randomized to receive brensocatib (n=8/dose) or placebo (n=5). PK evaluation showed rapid absorption, dose-dependent exposure, moderate elimination rate, and moderate exposure accumulation at steady state. Concomitant CFTR modulator use had no effect on absorption and disposition of brensocatib. Brensocatib exposure in CF pts was comparable with that of healthy subjects and NCFBE pts (Figure). In general, brensocatib was well tolerated and comparable based on CFTR modulator use and CF status.
Conclusion
Irrespective of CFTR modulator use, brensocatib exposure is comparable among healthy, CF, and NCFBE subjects, with a similar safety profile to previous trials.