Abstract
Background: Airway remodeling leads to irreversible airway obstruction and accelerated lung function decline in COPD.
Objective: To explore the conclusive role of Peroxisome Proliferator-Activated Receptor-? (PPAR?) in alveolar macrophages (AMs) polarization homeostasis in COPD and its underlying mechanism.
Methods: Bronchoalveolar lavage fluid (BALF) samples were collected from controls and COPD patients. Primary AMs, PPAR?-/- AMs, airway smooth muscle cells (ASMCs) and airway epithelial cells (AECs) were employed to dissect the role of PPAR? in vitro. Wild-type, TLR4-/- and IL-4-/- mice exposed to cigarette smoke (CS) were used in vivo experiments.
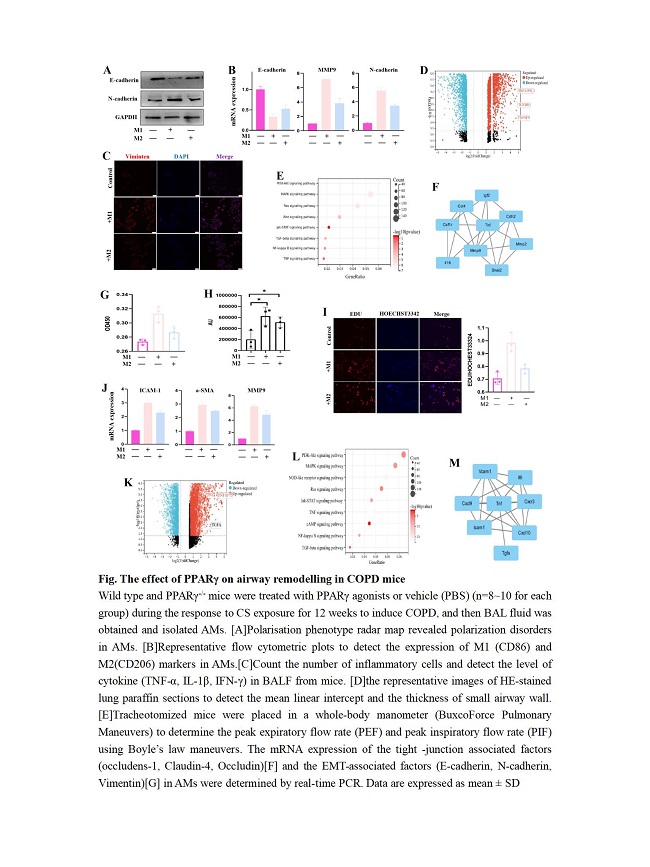
Results: Elevated percentage of M1-AMs and the expression of functionally cytokines was found in COPD patients, and were closely related to the disease severity. PPAR? expression in M2-type AMs was markedly increased in COPD patients. PPAR? promoted AMs polarization towards M2-type by inhibiting the JAK-STAT, MAPK and NF-?B pathways. M1/M2-AMs promoted EMT and airway smooth muscle proliferation in vitro and in vivo. PPAR? agonist significantly alleviated the airway remodelling of COPD mice.
Conclusions: Our research demonstrates that polarization homeostasis of AMs mediated
by PPAR? has the protective effect in airway remodeling, and may be a novel therapeutic target
for the intervention and treatment of airway remodeling in COPD.