Abstract
Introduction
Incidence of pneumoconiosis has recently increased in Australia, particularly silicosis and coal-workers pneumoconiosis. In IPF, anti-fibrotic agents like nintedanib slow disease progression and reduce frequency of acute exacerbations, but few data exist on pneumoconiosis.
Aim:
The NiPPs study aims to investigate the effect of nintedanib in progressive pneumoconiosis.
Methods:
Eligible patients were recruited for treatment with nintedanib 150mg PO BD for 3 years. The primary outcome measure was change in lung function measured by forced vital capacity (FVC) decline from baseline to end of study, as in IPF trials (INBUILD). Secondary outcome measures included K-BILD scores, other lung function parameters, CT progression, and 6 minute walk tests.
Results:
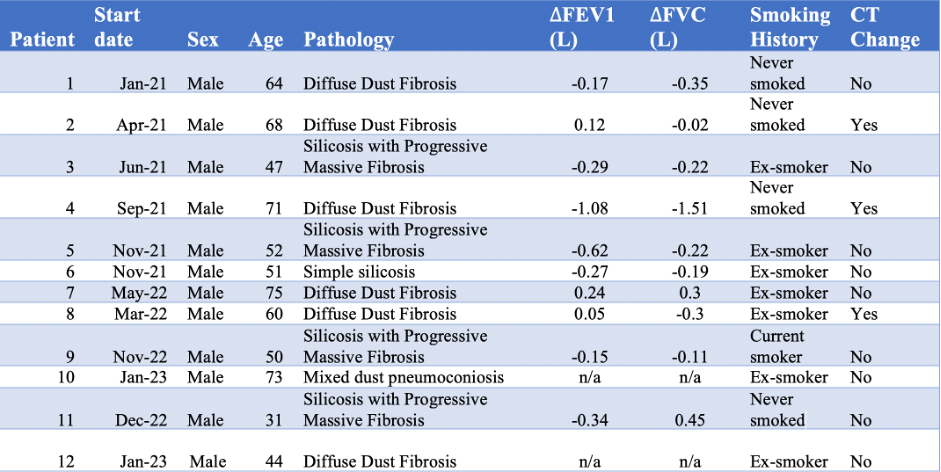
Recruitment started in 2019 but was suspended due to COVID-19 until late 2022. Of 89 patients screened, 12 patients were recruited; 10 remain successfully enrolled (all male, mean age 56 yrs (+/-14yrs); range 31-75 yrs). The average drop in FVC was 0.62mL/yr.
Conclusion:
Of 89 patients screened, a small number showed lung function deterioration similar to IPF. Patients with silicosis did not show lung function loss until late in disease, as has been previously described. Lung function using FVC is a not sensitive measure of progression in most types of pneumoconiosis and new methods of assessing progression are required.
Study sponsored by Boehringer Ingelheim.