Abstract
Background
Tezepelumab significantly reduced the annualized asthma exacerbation rate by 56% versus placebo in patients with severe, uncontrolled asthma in the phase 3 NAVIGATOR study (NCT03347279).
Objective
This post hoc analysis evaluated exacerbation characteristics in tezepelumab versus placebo recipients.
Methods
NAVIGATOR was a multicentre, randomized, double-blind, placebo-controlled study. Patients (12?80 years old) receiving medium- or high-dose inhaled corticosteroids and ?1 additional controller medication, with or without oral corticosteroids, were randomized 1:1 to tezepelumab 210 mg or placebo subcutaneously every 4 weeks for 52 weeks. Sites documented patient-reported symptoms, and medication use during exacerbations.
Results
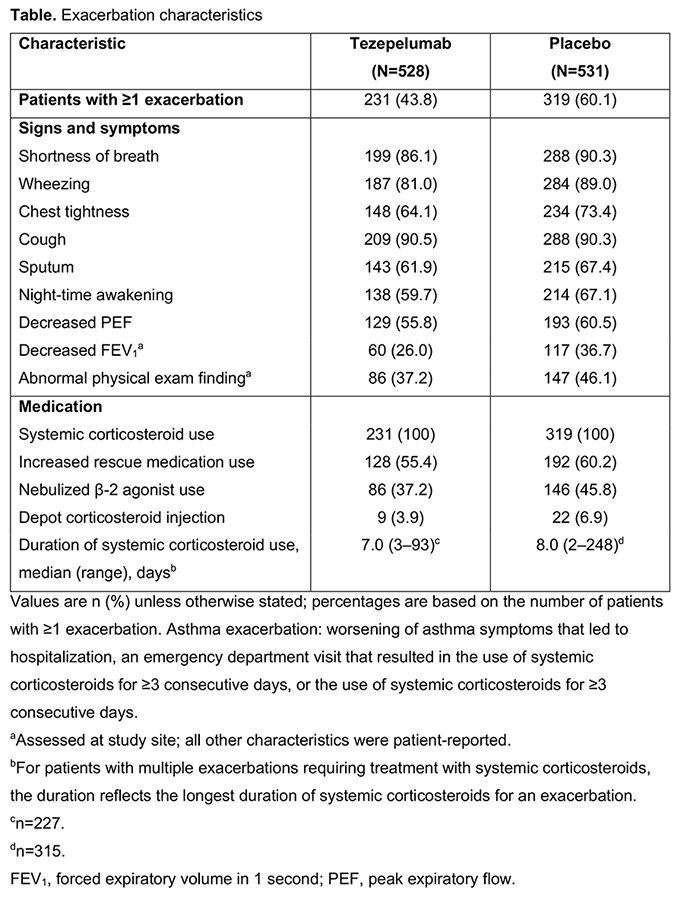
Of 1059 patients, 231/528 (43.8%) and 319/531 (60.1%) reported ?1 exacerbation in the tezepelumab and placebo groups, respectively. Most exacerbation symptoms occurred in slightly fewer participants receiving tezepelumab versus placebo (range of differences: 4.2?10.7%); cough was similar between groups. Medication use during exacerbations and the duration of systemic corticosteroid use was similar or slightly reduced for tezepelumab versus placebo (Table).
Conclusion
Compared with placebo, tezepelumab recipients had fewer exacerbations. When exacerbations occurred, symptoms and medication use were similar or slightly reduced among tezepelumab recipients.