Abstract
Objective: To describe frequency of serious adverse events (SAE) during treatment with mSTR for MDR/RR-TB under operational research (OR) conditions.
Methods: 250 MDR/RR-TB patients cohort with drug-susceptibility to fluoroquinolones and treated from 2019 with mSTR (bedaquiline, levofloxacin, linezolid, clofazimine and cycloserine/or delamanid for 39 weeks) was analyzed. SAE during therapy and 12 months follow-up were monitored, registered and managed.
Results: Baseline patient characteristics and frequency of SAE are presented in Table 1; types of SAE and action undertaken to manage SAE are presented at Diagrams 1 and 2. In total there were 43 SAE registered during therapy or 1.9 (95% CI 1.4 - 2.6) per 100 person-months. The criteria for SAE were: otherwise medically important - 23 (54%), hospitalization - 10 (23%), life-threatening - 4 (9%) and death - 6 (14%). Of 43 SAE, 30 (70%) were resolved, 4 (9%) resolving and 9 (21%) resulted in deaths. Of 9 SAE that resulted in deaths 2 (progressive coronary disease and heart failure) had possible relation to treatment, while 7 were assessed as not related to treatment: 3 ? cancer, 1 - severe open traumatic brain injury, 1 - HIV-associated encephalitis, 1- COVID-19, 1 - pulmonary hemorrhage.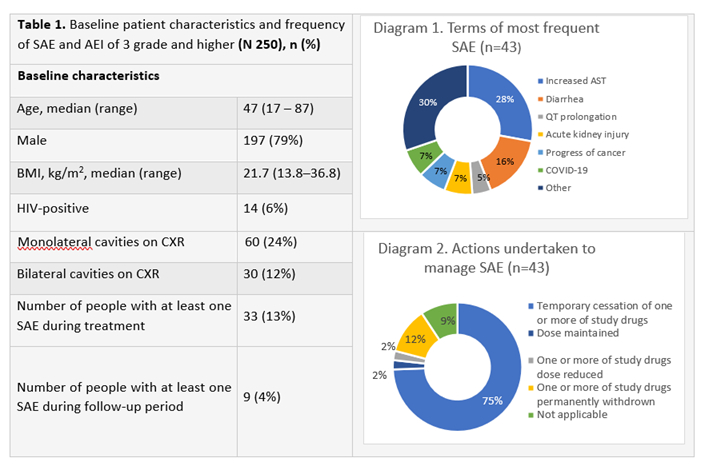
Conclusion: SAE occur during mSTR, but rarely lead to treatment discontinuation. Proper SAE monitoring and management are essential for any TB response.