Abstract
Introduction
Practical, validated patient diaries are needed to evaluate idiopathic pulmonary fibrosis (IPF) respiratory symptoms. EXACT®, a COPD symptom assessment patient-reported outcome (PRO) includes 11 items studied as separate subdomains in IPF known as Evaluating Respiratory Symptoms (E-RS?:IPF) (Bacci et al. Respir Med 2018).
Aims
To identify optimal EXACT®-based endpoints in IPF studies by reporting on EXACT® total score vs. E-RSTM cough and breathlessness subdomain scores in the CANAL cough trial (NCT04030026).
Methods
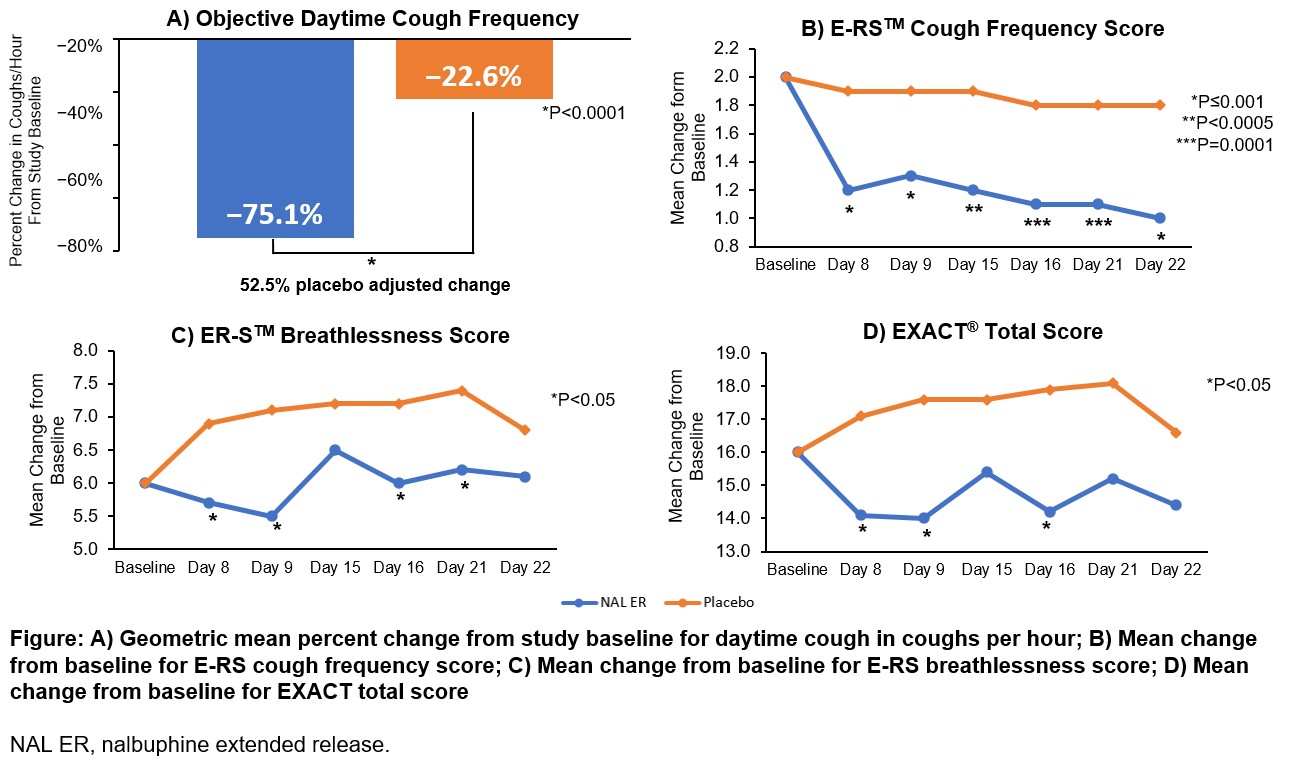
CANAL was a double-blind, placebo-controlled crossover phase 2 trial, investigating the antitussive effect of nalbuphine extended release (NAL ER) in IPF-related cough. The primary endpoint was mean change in daytime cough frequency, with multiple EXACT®-based secondary endpoints.
Results
Change from baseline in daytime cough frequency was statistically significant vs. placebo (Figure). Consistently, the mean change from baseline in E-RSTM subdomain cough score was significantly lower for NAL ER at all timepoints. Breathlessness subdomain and EXACT® total scores were numerically lower for NAL ER at all timepoints, reaching significance on Days 8, 9, 16, 21 and Days 8, 9 and 16, respectively (Figure).
Conclusions
The change in the E-RSTM cough subdomain better reflects the primary endpoint cough frequency reduction vs. EXACT® total score and could be a useful PRO in future IPF studies.