Abstract
Background
In a Phase II trial, treatment with the preferential PDE4B inhibitor BI 1015550 prevented a decline in FVC over 12 weeks in patients with idiopathic pulmonary fibrosis (IPF), irrespective of background antifibrotic (AF) use. The effect was seen as early as 2 weeks after treatment initiation.
Aims and objectives
To examine the possible contribution of bronchodilation to the treatment outcome, Phase II trial data were analysed to determine the effect of BI 1015550 on the FEV1/FVC ratio.
Methods
147 patients with IPF (77% male) who were currently receiving a stable dose of AF or not receiving AF were randomised and treated with 18 mg BI 1015550 (n=97) or placebo (n=50) twice daily for 12 weeks. The adjusted mean change from baseline in the FEV1/FVC ratio was assessed through to Week 12.
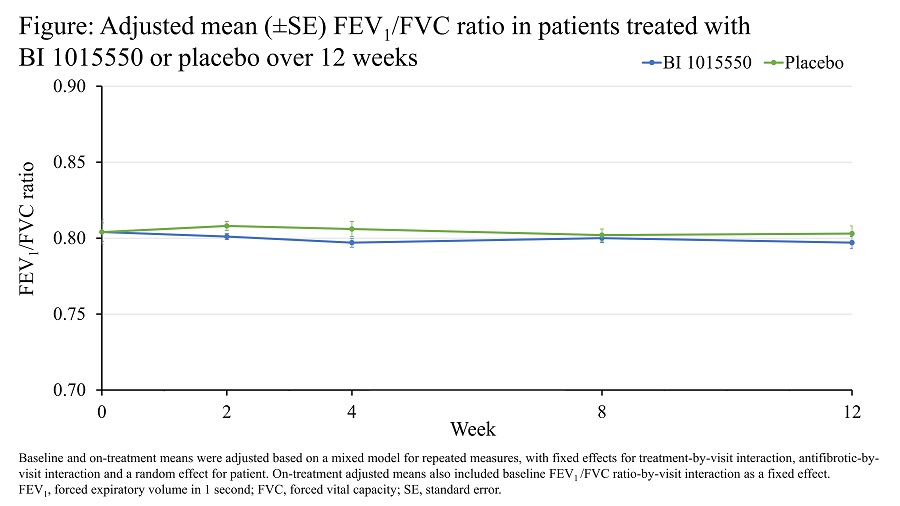
Results
No effect of BI 1015550 on the FEV1/FVC ratio was observed. At Week 0, the adjusted mean FEV1/FVC ratio was 0.804 in both groups. At Week 12, the adjusted mean change was -0.008 in patients receiving BI 1015550 and -0.001 in those receiving placebo (mean difference -0.006, 95% CI -0.018, 0.006, p=0.303). Findings were consistent in subgroup analyses based on AF status.
Conclusions
BI 1015550 had a significant treatment effect on FVC in patients with IPF, but it had no effect on the FEV1/FVC ratio. These functional data suggest that the treatment effect is not mediated by bronchodilation.