Abstract
Background: Improvements in patient-reported cough severity visual analog scale (VAS) scores have been reported through 52 weeks of treatment with gefapixant in two phase 3 studies (COUGH-1 and COUGH-2), but VAS changes earlier than 4 weeks have not been analyzed.
Aims and objectives: To assess changes in cough severity VAS over the first 4 weeks of treatment in COUGH-1 (NCT03449134) and COUGH-2 (NCT03449147).
Methods: Adults with refractory or unexplained chronic cough, cough duration ?1 year, and cough severity VAS ?40 mm (out of 100) were enrolled. This post hoc analysis of pooled data from participants who received gefapixant 45 mg BID or placebo assesses changes from baseline in weekly mean VAS scores, collected daily from Weeks 1-12, with a focus on the first 4 weeks of treatment.
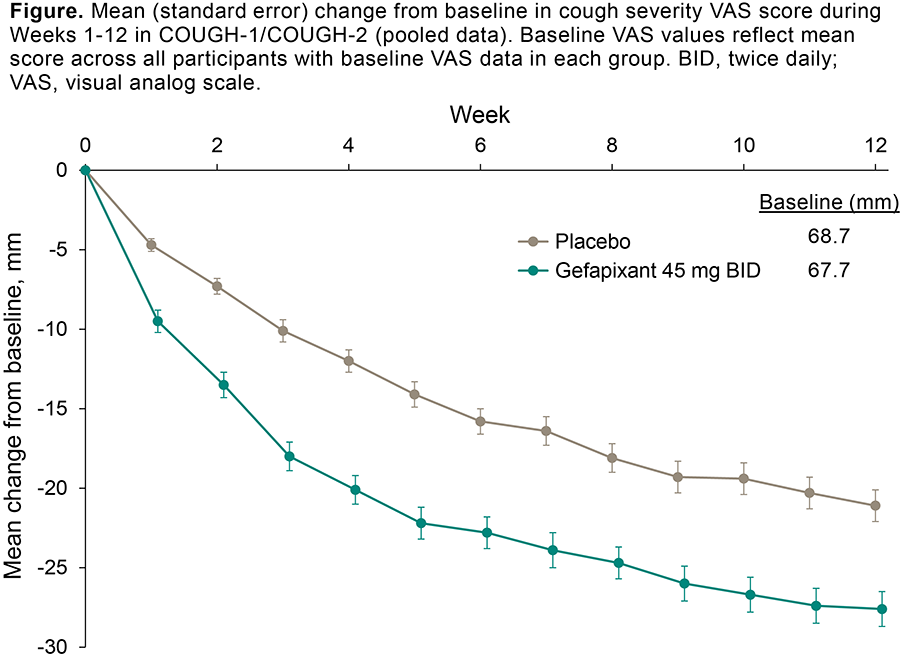
Results: A total of 682 and 678 participants were randomized to gefapixant or placebo, respectively. With gefapixant, most (73%) of the reduction in VAS was evident by Week 4 (-20.1 mm) compared with Week 12 (-27.6 mm; Figure). Weekly placebo-adjusted differences in change from baseline for gefapixant increased until Week 4 (-8.1 mm), and the placebo-adjusted reductions were sustained from Weeks 5-12 (range, -6.5 to -8.0 mm).
Conclusions: This analysis suggests most cough severity VAS improvements through 12 weeks of treatment with gefapixant 45 mg BID are observed within the first 4 weeks.