Abstract
Background: Stress urinary incontinence (SUI) is prevalent among women with chronic cough (CC); however, there are limited data on patient burden of cough-induced SUI (cSUI).
Aims and objective: To summarize baseline characteristics and cSUI burden among participants in a phase 3b trial of the P2X3-receptor antagonist gefapixant in cSUI (NCT04193176).
Methods: Adult women with diagnosed refractory or unexplained CC (RCC or UCC) ?12 months and SUI ?3 months were included. Additional criteria included ?40-mm cough severity visual analog scale (VAS) score (100-mm maximum), ?2 cSUI episodes/day, and positive screening SUI cough stress test. Episodes of cSUI were self-reported using the Incontinence Diary.
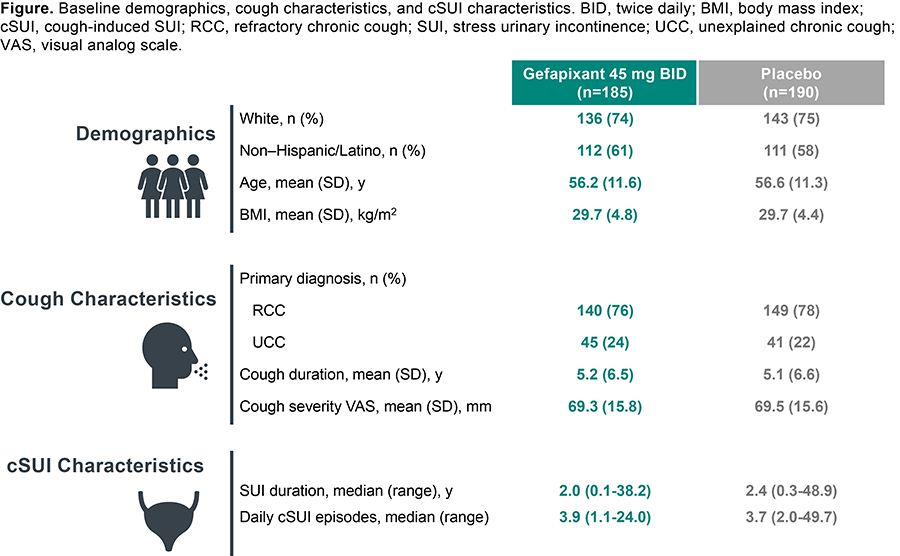
Results: Baseline characteristics were similar between groups who received gefapixant 45 mg BID (n=185) or placebo (n=190; Figure). Participants were predominantly White. Mean age was ~56 years and mean body mass index was 29.7 kg/m2. Most participants had a primary diagnosis of RCC. Mean cough duration was ~5 years and mean baseline weekly cough severity VAS score was ~69 mm. Participants had a median SUI duration of ~2 years and reported a median of ~4 daily cSUI episodes (7-day average).
Conclusions: Characteristics were similar between groups and consistent with prior RCC/UCC studies. These individuals experience multiple daily cSUI episodes, supporting the need for effective cough alleviation.