Abstract
Background: A phase 3b trial (NCT04193176) in refractory or unexplained chronic cough (RCC/UCC) and cough-induced stress urinary incontinence (cSUI) showed gefapixant 45 mg BID significantly reduced mean daily cSUI episodes after 12 wk vs placebo (pbo); however, the proportion with meaningful reductions in cough severity was not characterized.
Aims and objectives: To evaluate response to gefapixant using established clinically meaningful thresholds on the Cough Severity Diary (CSD; exploratory endpoint) in women with RCC/UCC and cSUI after 12 wk of treatment.
Methods: Women with RCC/UCC (CC duration ?12 mo), SUI (?3 mo), and ?2 daily cSUI episodes received gefapixant 45 mg BID or pbo. This post hoc analysis assesses ?1.3- and ?2.7-point improvements (ie, reductions) in CSD total score and mean CSD changes from baseline to Wk 12. The study was not powered for CSD comparisons.
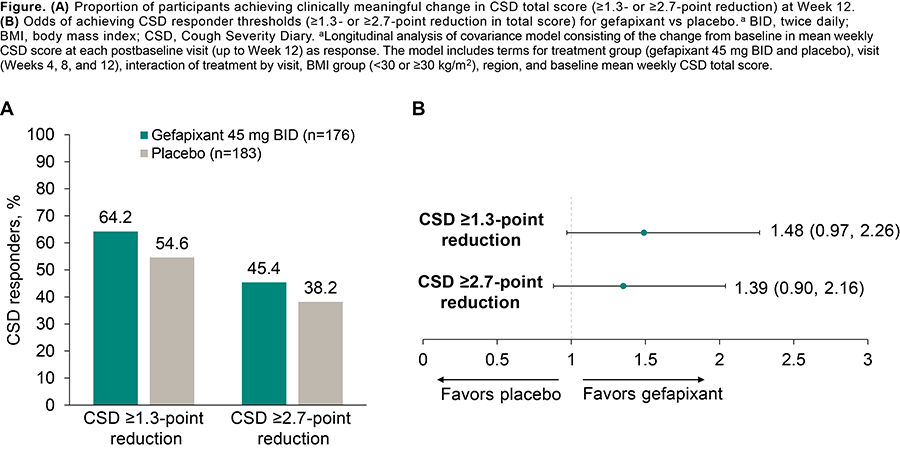
Results: Mean baseline CSD total scores were 5.84 (gefapixant, n=185) and 5.91 (pbo, n=190). A greater proportion receiving gefapixant had clinically meaningful ?1.3- and ?2.7-point reductions in CSD total score (Figure). Mean CSD changes from baseline at Wk 12 were greater for gefapixant vs pbo across total (-2.51 vs -2.05) and domain scores (frequency, -2.57 vs -2.05; intensity, -2.54 vs -2.11; disruption, -2.38 vs -1.98).
Conclusions: Gefapixant improved patient-reported cough severity in women with RCC/UCC and cSUI vs pbo.