Abstract
Background: Interferon beta (IFN-?), a key host antiviral mediator, can be suppressed by virus or host factors locally at the site of infection. Inhaled SNG001 (IFN-?-1a nebuliser solution) aims to restore lung IFN-? levels.
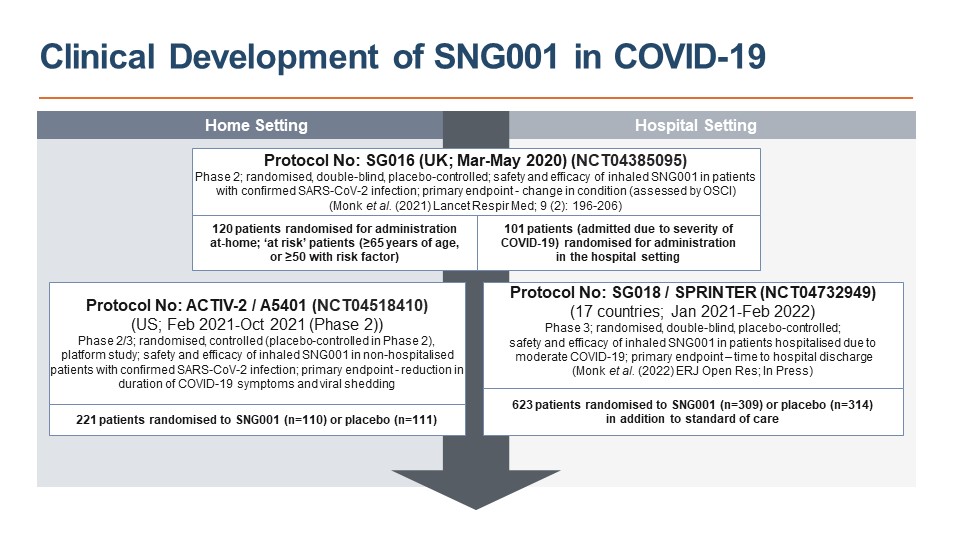
In a Phase 2 trial hospitalised COVID-19 patients receiving SNG001 were more than twice as likely to recover, supporting progression to the Phase 3 SPRINTER trial in hospitalised patients and inclusion of SNG001 in the ACTIV-2 Phase 2/3 trial in non-hospitalised volunteers (Figure 1).
The SPRINTER study did not meet its primary efficacy endpoints, hospital discharge and recovery, likely due to improved standard of care reducing the window to show a treatment effect. An encouraging signal for the key secondary endpoint, prevention of progression to severe disease or death (26% RRR;OR 0.71(0.44,1.15);p=0.161), was observed and supported by post hoc analyses of at risk groups.
Results: In ACTIV-2 there were no statistically significant differences between SNG001 and placebo for the primary outcomes of safety (SNG001 was well-tolerated), symptom resolution, or virology. However, fewer participants required hospitalisation following SNG001 treatment (N=1/110, [1%]) compared to placebo (N=7/110, [6%]), 86% RRR (P=0.07). IDSMB recommended progression to Phase 3.
Conclusions: SNG001 was well tolerated across trials and should continue to be investigated for COVID-19 in the home and hospital settings.