Abstract
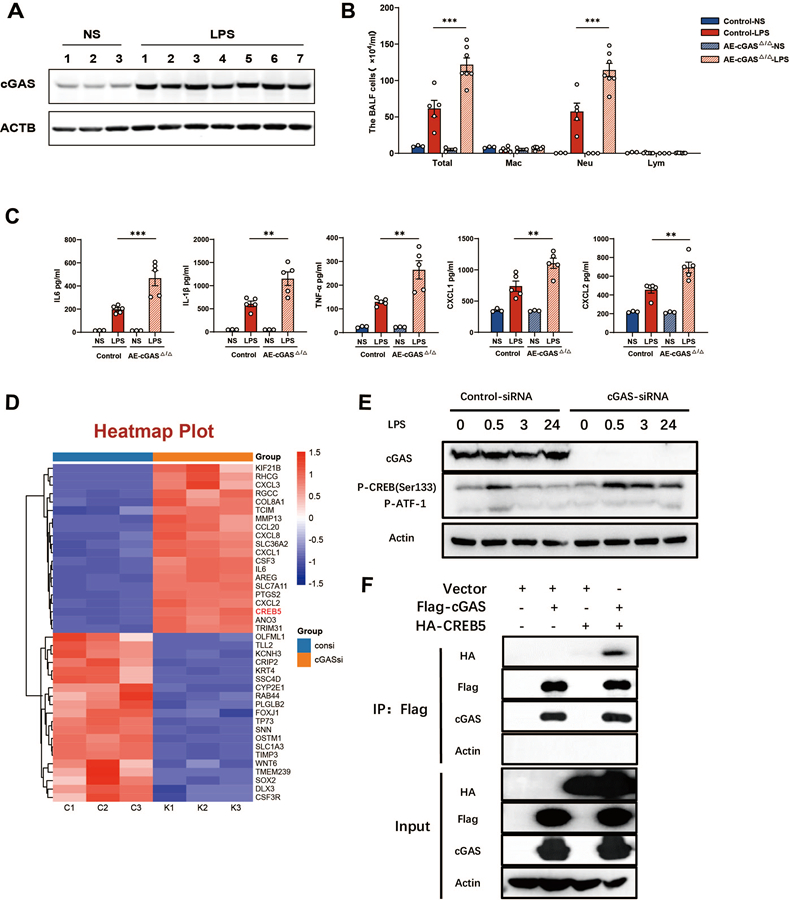
During severe infection-induced acute respiratory distress syndrome, invading microorganisms can cause damage to lung epithelium and promote the release of cytosolic DNA. However, the detailed mechanism of acute lung injury induced by increased cytosolic DNA from airway epithelium remains elusive. To address this question, airway epithelial cyclic GMP-AMP synthase (cGAS) was deleted in lipopolysaccharide(LPS)-induced acute lung injury mouse model as well as human bronchial epithelial (HBE) cells. We observed that LPS triggered cytosolic DNA release to activate cGAS. Conditional deletion of airway epithelial cGAS significantly exacerbated pulmonary inflammation, vascular permeability, pulmonary edema and mortality in LPS-induced acute lung injury mouse model. In HBE cells, knockdown of cGAS deteriorated LPS-induced production of IL-6 and IL-8. Mechanically, deletion of cGAS augmented phosphorylated CREB expression, and cGAS could directly interacted with CREB via its C-terminal domain. Furthermore, CREB knockdown rescued LPS-induced excessive inflammatory response caused by cGAS deletion. Our study for the first time demonstrated that airway epithelial cGAS played a protective role in LPS-indued acute lung injury, and confirmed a non-canonical cGAS-CREB pathway which specifically regulated the inflammatory responses in airway epithelium.