Abstract
Background: Oral imatinib showed efficacy in pulmonary arterial hypertension but high oral doses were limited by frequent adverse events (AEs). Targeted administration of low-dose AER-901 to the lungs may have similar efficacy and an improved AE profile.
Aim: A randomized, double-blind, placebo-controlled, single- and multiple-ascending dose (SAD and MAD) phase 1 study evaluated safety, tolerability, and pharmacokinetics (PK) of AER-901 in healthy adults (NCT04903730).
Methods: The SAD (5-80 mg), MAD (10-40 mg daily x7 days), and PK of 40 mg AER-901 vs 100 mg oral imatinib were evaluated.
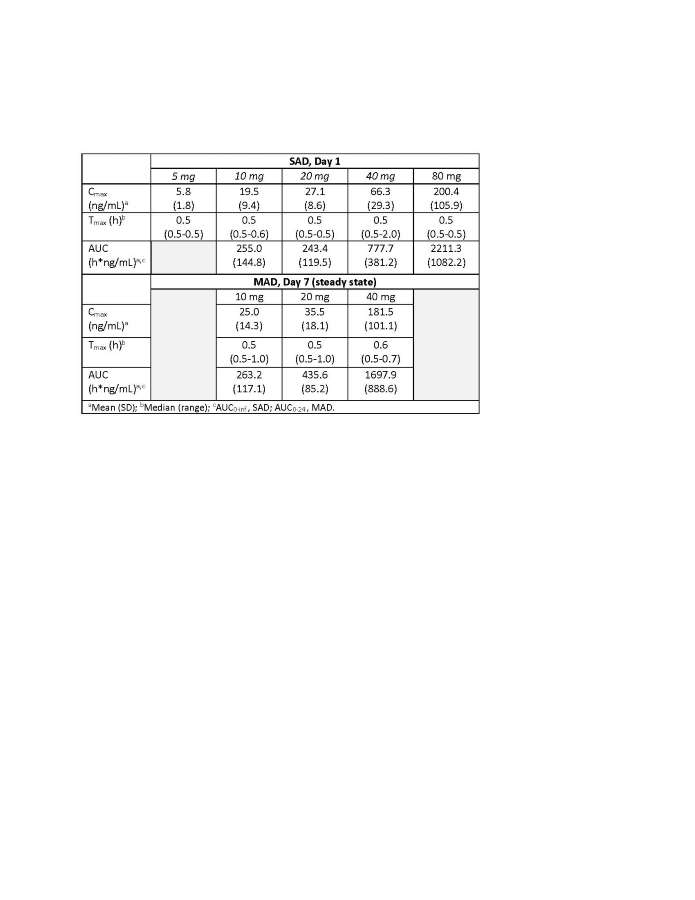
Results: 84 participants were enrolled. Key AER-901 PK parameters are shown in Table. Peak plasma concentrations occurred rapidly after single and multiple doses, with lower systemic exposure compared to oral imatinib. There was no substantial accumulation after multiple doses. AEs were generally mild and consistent with inhaled products; there were no treatment-related serious AEs.
Conclusions: Targeted administration of AER-901 to the lung was generally well tolerated and associated with low systemic imatinib exposures. Evaluation of AER-901 in patients with pulmonary hypertension in phase 2 is warranted.