Abstract
Background
Nintedanib was approved in the UK for use in progressive fibrosing interstitial lung disease (PF-ILD) in November 2021.1 The prescribing criteria defines PF-ILD using the diagnostic criteria established by the INBUILD study.2 There has been no national evaluation of the use of nintedanib for PF-ILD in a real-world setting.
Methods
A service evaluation was distributed to specialist interstitial lung disease (ILD) centres in the UK in September 2022. Local clinical audit department approval was obtained.
Results
24 centres responded to the service evaluation invitation. Between 17/11/2021 and 30/09/2022, 1120 patients were commenced on nintedanib for PF-ILD. The most common diagnoses were hypersensitivity pneumonitis (298/1120,26.6%), connective tissue disease ILD (197/1120,17.6%), rheumatoid arthritis associated ILD disease (180/1120,16.0%), idiopathic non-specific interstitial pneumonia (125/1120,11.1%) and unclassifiable idiopathic interstitial pneumonic (100/1120,8.9%). Figure 1 demonstrates dual use of immunosupression and anti-fibrotic therapy. 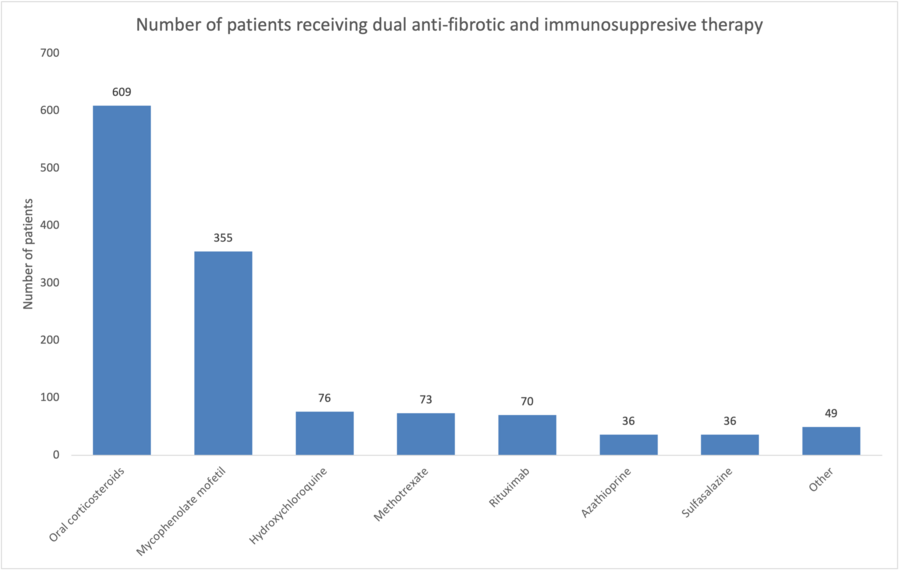
Discussion
This real-world service evaluation demonstrates widespread use in the UK of nintedanib for a broad range of PF-ILD subtypes. Contrary to the INBUILD study, clinicians are commonly using combined immunomodulatory and anti-fibrotic therapy.2
1. NICE, England, 2021.
2. Flaherty K.R. et al. N Engl J Med.2019;381(18):1718-1727.