Abstract
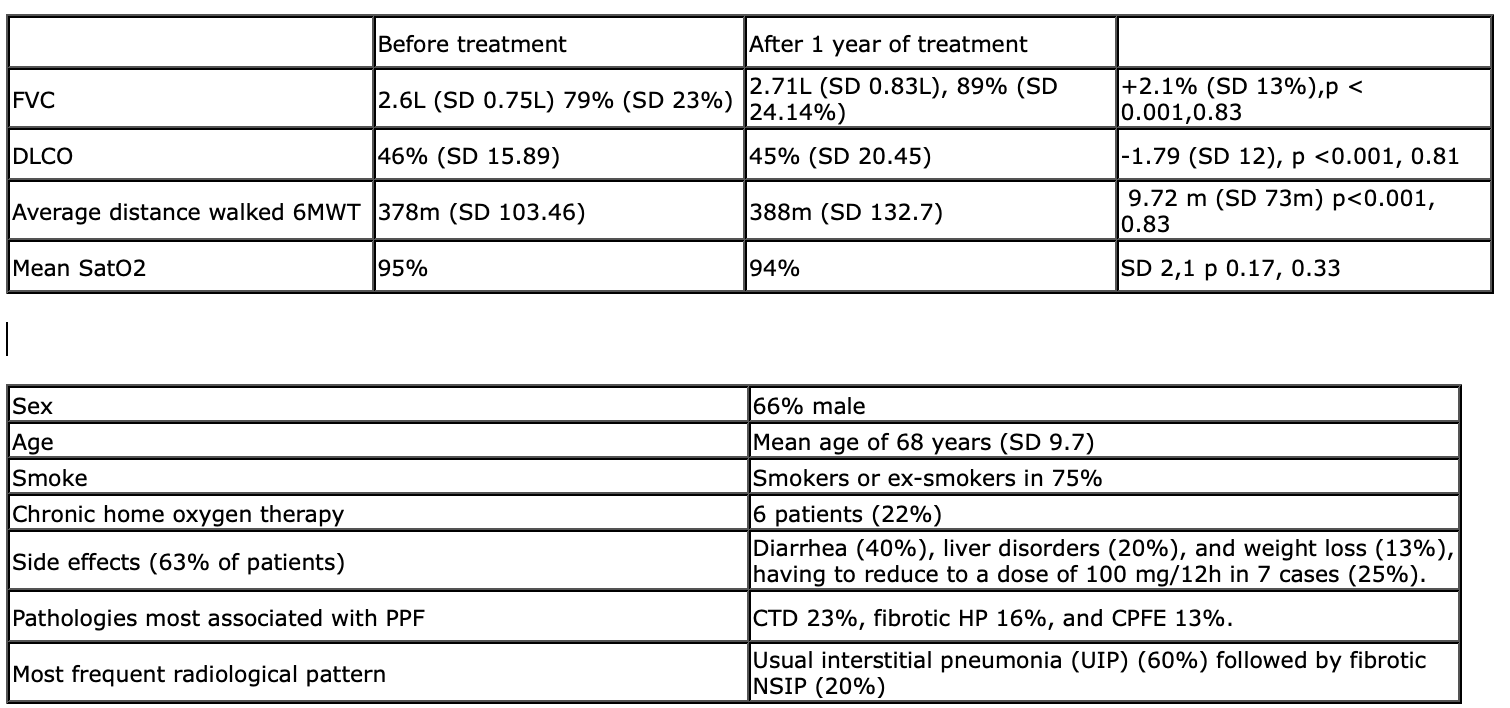
The current ATS/ERS/JRS/ALAT clinical practice guideline recommends the use of Nintedanib for the treatment of PPF. Primary endpoint: To evaluate the efficacy of Nintedanib to slow down the loss of lung function in patients with PPF. Secondary endpoints: to evaluate clinical changes and the tolerance to the drug, and the most prevalent side effects. Methods:Multicenter prospective observational study with the participation of the Hospital La Ribera and the General Hospital of Castellón.27 patients were recruited with a diagnosis of non-IPF PPF associated with connective tissue disease (CTD), pneumoconiosis, hypersensitivity pneumonitis(HP), idiopathic non-specific interstitial pneumonia (NSIP), unclassifiable interstitial pneumonia, and combined pulmonary fibrosis and emphysema (CPFE) for which Nintedanib was started between Nov'20 and May'22 150mg/12h.We compared the variables FVC, DLCOc, Six Minutes Walking Test (6MWT) collecting them the month previous to the treatment start and one year after.Results (figure1)Conclusions:In our study nintedanib did slow and stabilize the decline of FVC&DLCO. 6MWT improved with an increase in the distance walked without being associated with greater desaturation in satO2. Most patients suffered gastrointestinal side effects that could be managed with diet changes and temporary discontinuation of nintedanib, but in a high number of cases a drug dose reduction was required