Abstract
Introduction
Oral anti-fibrotics have been shown to attenuate lung function decline in PPF however side-effects, often gastrointestinal (GI), are common. Nebulised pirfenidone achieves a 35-fold higher peak epithelial lining fluid concentration and 1/15th of the systemic exposure than standard dose oral pirfenidone, with less frequent side effects and stabilisation of FVC in phase Ib study (West A, et al. Thorax 2023, accepted for publication).
Methods
A total of 28 patients with non-IPF PPF, and no treatment alternatives, were recruited to an ongoing phase II open-label extension trial of nebulised pirfenidone solution at 22 sites in 6 countries. FVC data at 48 weeks was available for 18 patients with PPF between June 2021 and February 2023.
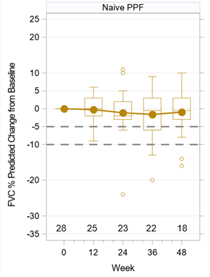
Results
Median treatment duration for patients with PPF was 374 days. The mean±SD change in FVC from baseline at 48 weeks was -31.7±212.4 mls. The mean change in % predicted FVC was -0.94% (SD 6.58). Adverse effects of special interest included respiratory tract infection, cough, and rash. Only 1 patient (3.6%) reported GI side effects with this medication.
Conclusion
This is the first study of nebulised pirfenidone in patients with PPF and suggests nebulised pirfenidone may be both well-tolerated and effective, and further investigation is warranted.