Abstract
Background: Benralizumab is an add-on maintenance therapy for severe eosinophilic asthma (SEA).
Objective: To describe clinical outcomes for SEA patients treated with benralizumab, stratified by prior biologic use.
Methods: This study was a retrospective chart review, with data from 14 sites across Canada (2017-2020). Index date was defined as benralizumab initiation. Eligible SEA patients were at least 18 years old, received at least one benralizumab injection, had a minimum one year of data prior to index (baseline) and a minimum 3-month follow-up. Annual exacerbation rate (AER), blood eosinophil count (BEC) and lung function were assessed at baseline and 48 ± 8 weeks post-index.
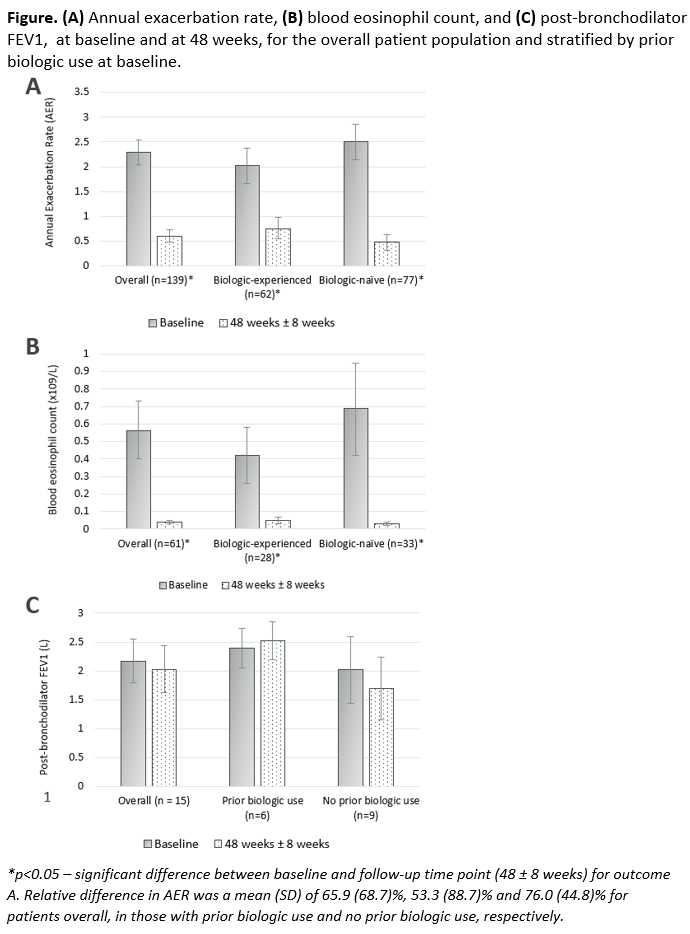
Results: Of 306 patients included in this study, 144 (47.1%) were female and mean (standard deviation, SD) age at index was 57.5 (14.1) years. 137 patients (44.8%) were biologic experienced (mepolizumab n=95, omalizumab n=74, reslizumab n=10) and 54.2% were biologic naïve. At 48 weeks, 8.5% of patients discontinued benralizumab (32% due to lack of efficacy). There were significant reductions in AER and BEC, and sustained post-bronchodilator FEV1, at 48 weeks, regardless of prior biologic use (Figure).
Conclusions: Significant reductions in exacerbations and BEC, and preserved lung function were observed at 48 weeks post benralizumab initiation for both biologic-experienced and biologic-naïve patients with SEA.