Abstract
Background
When prescribing a biologic in severe asthma, it is necessary to understand the impact of baseline patient (pts) characteristics, including eosinophils (eos).
Aims and objectives
To describe pts characteristics and one-year clinical outcomes in ORBE II study pts based on initial blood eos count (BEC).
Methods
ORBE II (NTC04648839) is a real-world retrospective multicenter study conducted in severe eosinophilic asthma pts treated with benralizumab in Spain. Pts were split into two groups according to their BEC (low eos [<300 eos/µL] and high eos [?300]).
Results
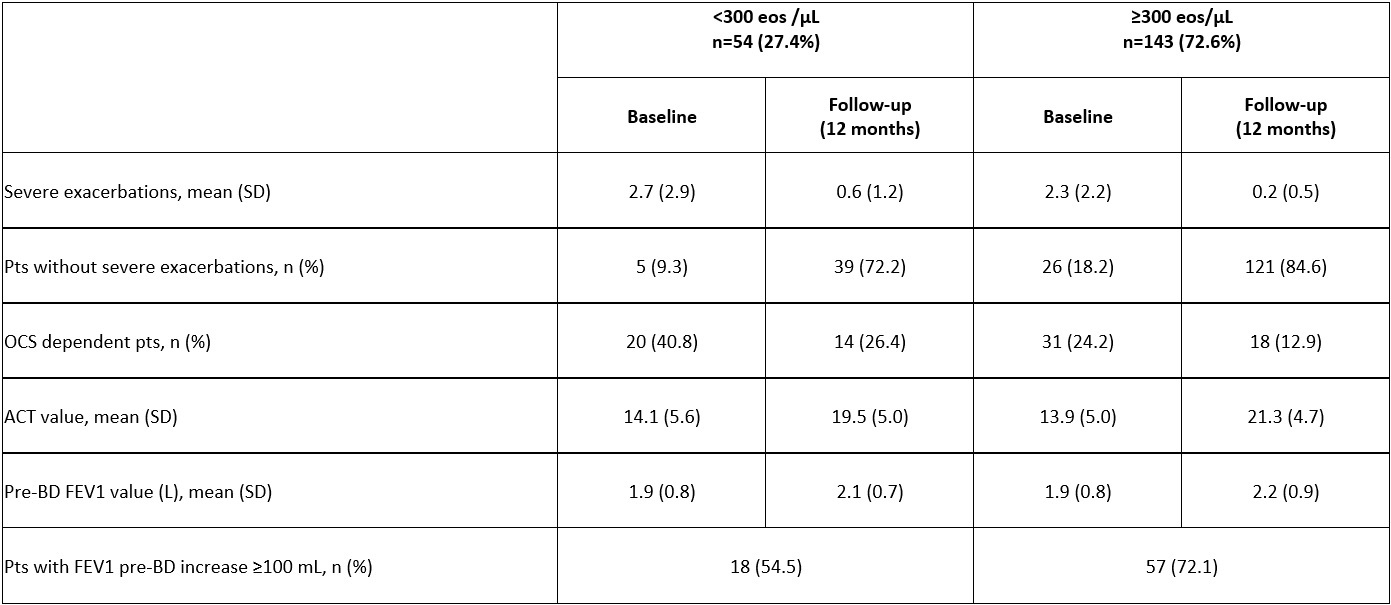
High eos pts had more T2 inflammation (higher FeNO and IgE) and presence of allergic asthma and comorbid nasal polyposis. Low eos pts presented more exacerbations and hospitalizations and a similar % of corticosteroid dependence. An important reduction of severe exacerbations, emergency room visits, and hospitalizations, and an improvement in ACT and lung function was observed in both groups, with a more pronounced effect in high eos pts (Table).
Conclusions
These results support the effectiveness of benralizumab in pts regardless of their baseline BEC.