Abstract
Introduction: Baseline (BL) demographics and disease characteristics are associated with asthma disease severity. TRAVERSE (NCT02134028) evaluated the long-term safety and tolerability of dupilumab (DPL) in adult and adolescent patients (pts) with asthma who participated in a previous DPL asthma study. Safety was consistent with the known DPL safety profile.
Objective: Assess the long-term efficacy of DPL during TRAVERSE in subgroups defined by parent study (QUEST) BL demographics and disease characteristics.
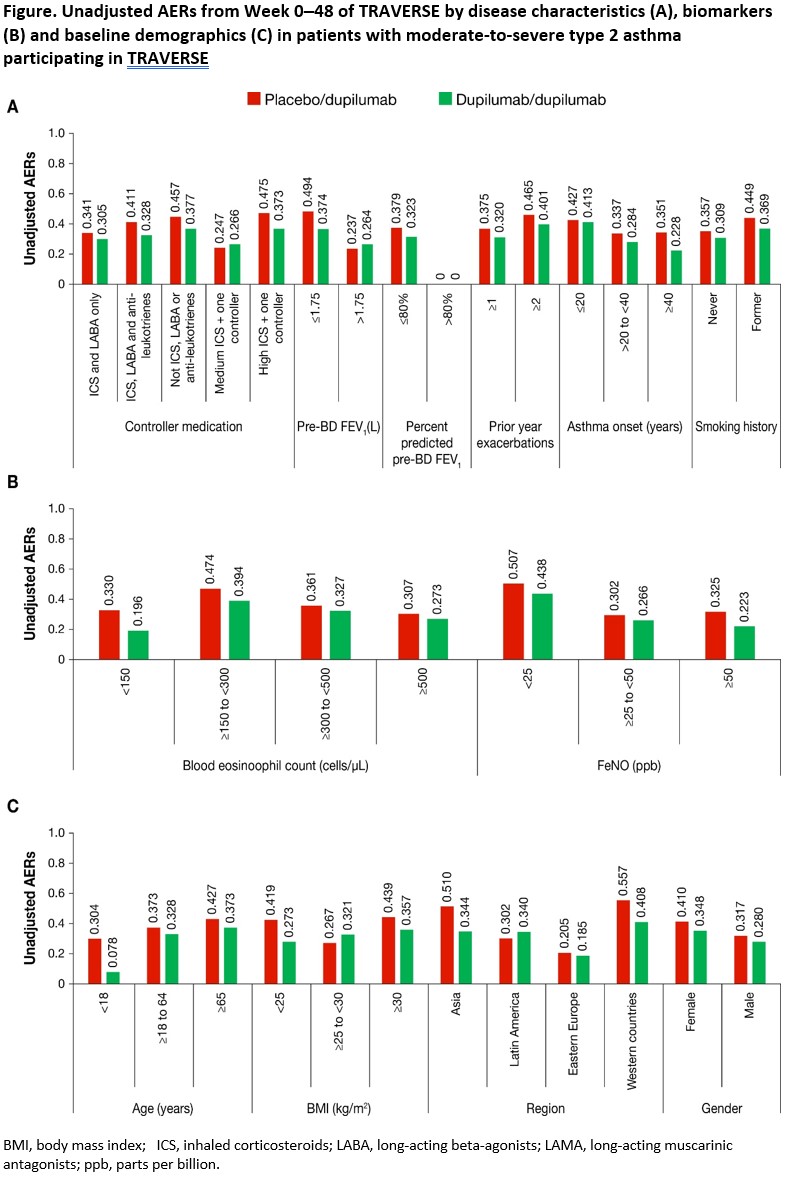
Methods: Annualized severe exacerbation rates (AER) during TRAVERSE (Wk 0?48) and change from QUEST BL in pre-bronchodilator FEV1 (L) at Wk 48 were assessed in pts with moderate-to-severe type 2 asthma (blood eosinophils ?150 cells/µL or fractional exhaled nitric oxide [FeNO] ?25 ppb) from QUEST (52 weeks) who enrolled in TRAVERSE, further stratified by BL demographics and disease characteristics (Figure).
Results: 1,227 pts from QUEST enrolled in TRAVERSE (placebo/DPL: 423; DPL/DPL: 804). DPL sustained low AERs throughout TRAVERSE across subgroups (Figure). Similarly, improvements in pre-bronchodilator FEV1 were sustained throughout TRAVERSE.
Conclusions: DPL treatment reduced exacerbations and improved lung function up to 48 weeks in TRAVERSE in pts with moderate-to-severe type 2 asthma across subgroups stratified by QUEST BL demographics and disease characteristics.