Abstract
Introduction: Dupilumab reduces rates of asthma exacerbations and improves lung function in children (VOYAGE, NCT02948959) and adults and adolescents (QUEST, NCT02414854) with uncontrolled, moderate-to-severe asthma. Previous data have demonstrated that fractional exhaled nitric oxide (FeNO) is a valid prognostic biomarker, a predictor of response to dupilumab.
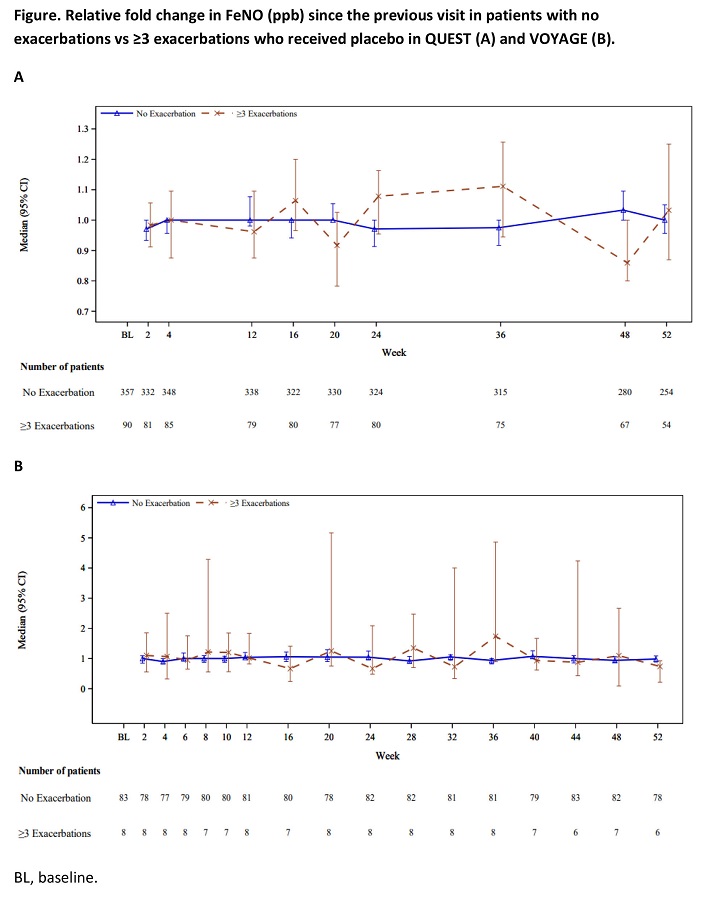
Aims and objectives: To evaluate the stability of FeNO over time using standard American Thoracic Society (ATS)/European Respiratory Society (ERS) FeNO guidelines in both therapy and placebo patients from QUEST and VOYAGE studies.
Methods: FeNO levels were collected in placebo patients (pts) from QUEST (?12 years) and VOYAGE (6?11 years), in the overall population and in non-exacerbating (0 baseline annualized severe exacerbation rate [AER]) and frequent exacerbator (>= 3+ AER at baseline) subpopulations.
Results: Median (95% CI) FeNO at baseline in non-exacerbators and frequent exacerbators was 25.0 (22.0, 27.0)/34.0 (27.0, 40.0) in QUEST, and 18.0 (14.0, 21.0)/48.5 (9.0, 100.0) in VOYAGE. At Week 52, fold change in FeNO from the previous visit was 1.00 (0.96, 1.05)/1.03 (0.87, 1.25) for 0 and >=3+ AER at baseline patients in QUEST, and 0.98 (0.89, 1.09)/0.74 (0.22, 0.92), in VOYAGE (Figure).
Conclusions: Variability of FeNO measurements was low and stable in between visits among exacerbator and non-exacerbator patients enrolled in QUEST and VOYAGE.