Abstract
Introduction: New biologics for severe eosinophilic asthma (SEA) significantly reduce oral corticosteroid (CS) exposure with the potential to reduce side effects from CS toxicity. We have previously reported CS toxicity using the glucocorticoid toxicity index (GTI) after 1yr biologics.(1)
Aim: Longitudinal assessment of CS-toxicity reduction in SEA patients after 3yrs biologics to assess longer term CS reduction and trajectory of CS-related toxicity.
Methods: 89 patients with SEA had repeat assessment of CS related toxicity 3yrs after biologic initiation using the GTI.(2) The GTI aggregate improvement score (AIS) is a bidirectional measure of total toxicity (minimal important difference (MCID) ?-10).
Results: Compared to yr1, median cumulative annual prednisolone dose continued to decrease (2518.5 v 292 mg/year at yr3, p<0.001); improvement in PROMS was maintained but did not further improve at yr3.
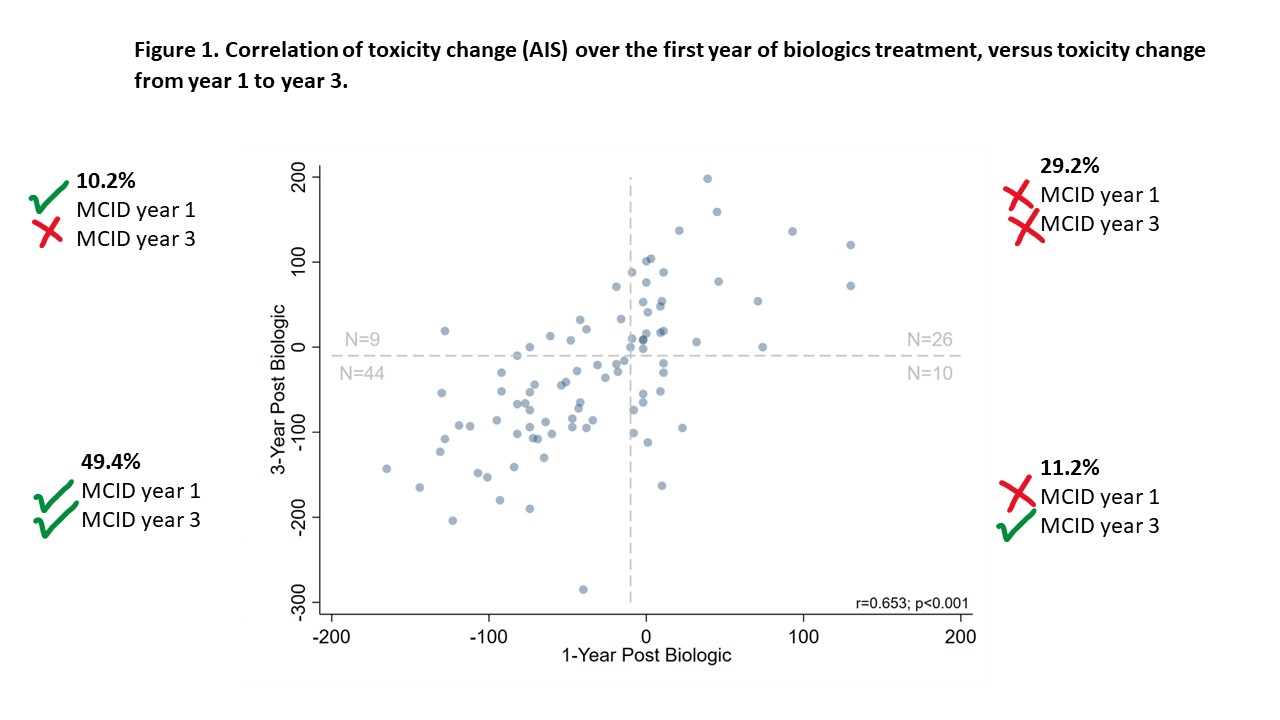
Median(IQR) AIS at 3yrs was -36 (-94, 19); 61% (54/89) met the MCID for toxicity improvement. Lack of toxicity improvement at yr1 predicted non-improvement at yr3 (30% of cohort, Figure1). ~80% of those that met MCID at yr1 met MCID yr3.
Conclusion: Toxicity assessment at 1yr predicts ongoing toxicity change; ~1/3 of patients do not have a significant toxicity reduction after 3yrs biologics questioning if biologics are introduced too late for this group.
1.Eur Respir J 2022; 59: 2100160
2. J Allergy Clin Immunol Pract 2021;9(1):365-372