Abstract
Rationale: Methacholine challenge testing (MCT) is used in patients with suspected asthma but normal spirometry. Prior research (Montfort Hospital) has led to the development of a clinical prediction model (CPM) for MCT results in patients (2016-2018) with normal spirometry (Chest 2021;160(4):A2159). We aimed to perform a temporal validation at our institution of this CPM.
Table 1. CPM
| BMI | <25/25-29/30-39/?40 |
| Gender | Male/Female |
| FEV1% predicted | <90/90-99/100-109/?110 |
| FEV1 reversibility (12%/200mL) | Yes/No |
| FEF25-75% predicted | ?/>120 |
| FEF25-75 % reversibility | <20/20-39/?40 |
Methods: 289 patients (?14 years old) with baseline normal spirometry underwent MCT (July 2020-June 2022). Predicted risk for each subject was determined using the CPM. We defined an abnormal MCT if PC20?16 mg/mL. Brier score was measured, in addition to Hosmer-Lemeshow (HL) test (calibration) and receiver operating characteristic area under the curve (AUC); analyses were also repeated for subjects with <5% predicted risk.
Results: Mean predicted risk was 9.6%; 7.3% (21/289) had PC20?16 mg/mL. 30.1% (87/289) had a predicted risk <5% (mean risk 3.1%); 2.3% (2/87) with PC20?16 mg/mL. Brier score was 0.07, with HL p=0.35; AUC was 0.63. For subjects with a predicted risk <5%, Brier score was 0.02 and HL p=0.39.
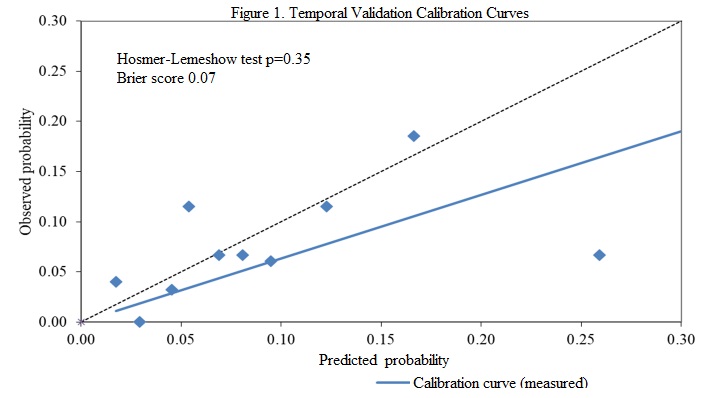
Conclusions: Temporal validation revealed excellent calibration of this CPM. The CPM could be very useful to identify subjects at low risk of abnormal MCT.