Abstract
Background:The 3TR consortium has proposed a composite response variable, the Core Outcome Measures for Severe Asthma (COMSA, Khaleva et al. ERJ 2022). The COMSA strategy was tested in the mepolizumab arm of the BIOCROSS real-life study of biologics in severe asthma.
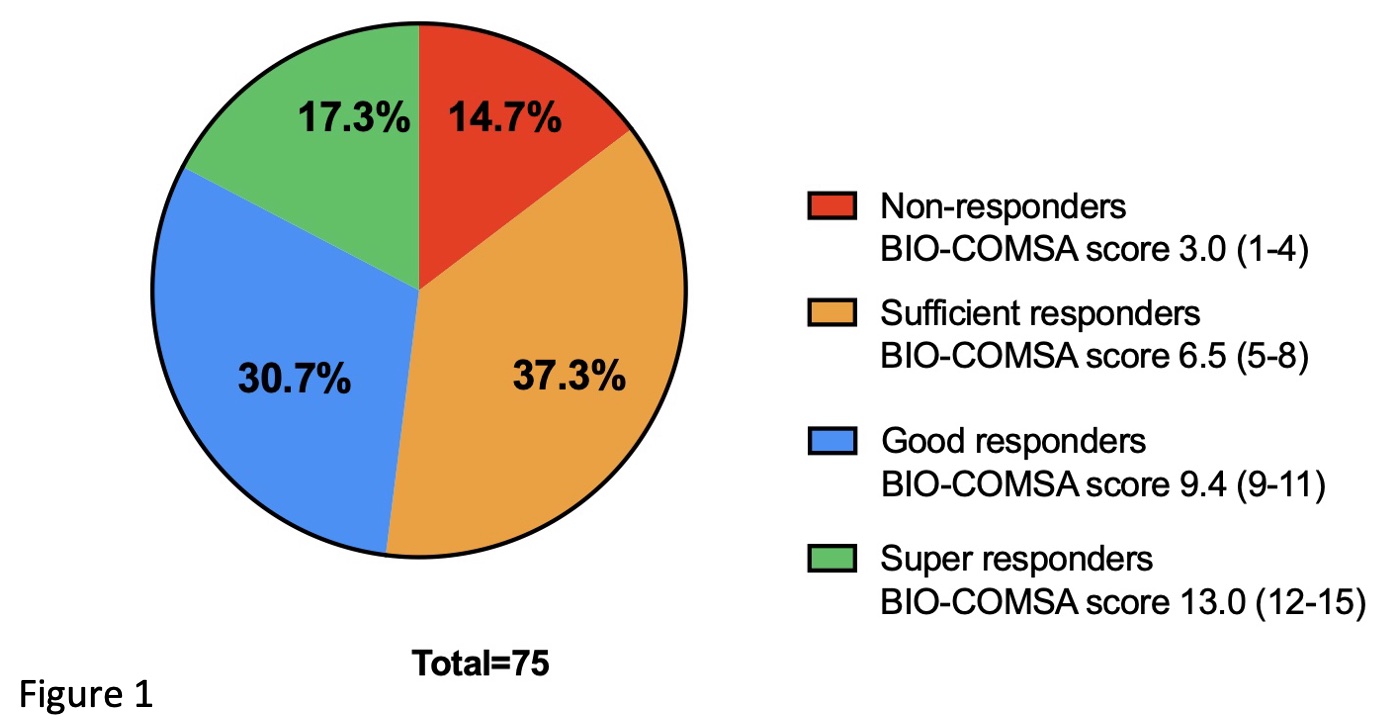
Methods: 75 patients with severe eosinophilic asthma diagnosed according to ERS/ATS criteria were examined before treatment and after 4,12, and 24 months. The changes in five outcomes [Asthma Control Questionnaire-5 (ACQ-5); Asthma Quality of Life Questionnaire (AQLQ); Forced Expiratory Volume in 1 second (FEV1), exacerbations and maintenance dose of oral corticosteroids (mOCS)] were scored after one year using a scale of five points in each domain. The sum of the five score was used to classify the response with 15 points being maximal improvement.
Results: For distinct levels of treatment response were identified (Figure 1) with great contrast between the super-responders (mean score 13) and non-responders (mean score 3). FEV1 increased by 471±459 mL and ACQ-5 decrease by 2.25±1.46 points among the super-responders as compared with 57±250 mL decrease and 0.05 ± 0.99 point difference in the non-responder group (p<0.01 for both outcomes). In contrast, blood eosinophils were reduced to the same extent in both groups.
Conclusion: The COMSA strategy was able to identify super-responders and non-responders to treatment with mepolizumab.