Abstract
Context: In severe asthma (SA), a history of exacerbations is associated with a poorer prognosis. Mepolizumab reduces clinically significant asthma exacerbations (CSEs) and maintenance oral corticosteroid (mOCS) use in SA.
Aim: To assess the impact of prior exacerbation history on mepolizumab outcomes in SA.
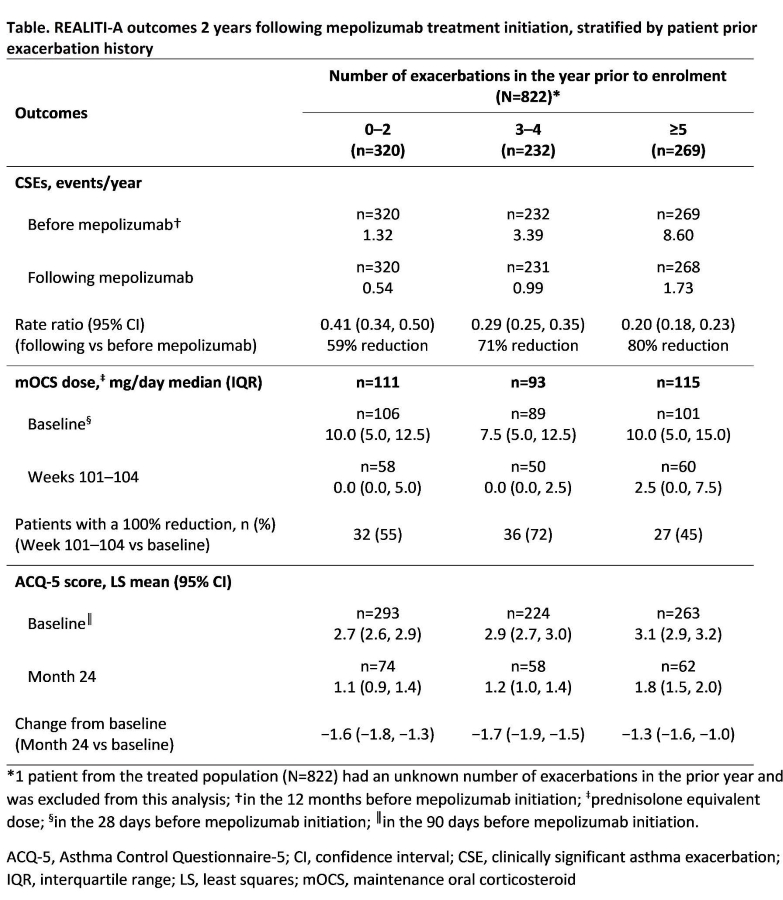
Methods: REALITI-A, a 2-year international, prospective study, enrolled adults with asthma newly prescribed (physician?s discretion) mepolizumab 100 mg subcutaneously (index). Data were collected 12 months pre- and 24 months post-index. This post hoc analysis assessed outcomes grouped by exacerbation history (0?2, 3?4 and ?5 exacerbations in the year before enrolment) and included the rate of CSEs (requiring systemic corticosteroids and/or an emergency department visit/hospitalisation) pre- and post-index, change from baseline (28 days before index) in daily mOCS dose at Weeks 101?104, and change from baseline (90 days before index) in Asthma Control Questionnaire (ACQ)-5 score at Month 24.
Results: All outcomes improved post- vs pre- index, regardless of exacerbation history (Table). The rate of CSEs reduced by 59?80%. At Week 101?104, median mOCS dose reduced by 75?100%; 45?72% of patients discontinued mOCS. At Month 24, least squares mean ACQ-5 scores improved by 1.3?1.7 points.
Conclusions: Real-world mepolizumab therapy benefits patients with SA, regardless of exacerbation history.
Funding: GSK (204710)