Abstract
Context: In clinical and real-world studies, mepolizumab is well tolerated with a favorable benefit-risk profile in patients with severe eosinophilic asthma (SEA); longer-term real-world safety data are required.
Aim: To report the long-term safety profile of mepolizumab in SEA.
Methods: This open-label, multicenter, Phase IIIb study enrolled patients with severe asthma who previously partook in a mepolizumab clinical study (<6-month gap). Patients received 4-weekly add-on mepolizumab (physician discretion) subcutaneously (SC: 100mg, ?12years; 40mg/100mg [by body weight], 6?11years) every 4 weeks until commercial licensing/August 2022. Adverse events (AEs) data were collected up to 28 days after the last mepolizumab dose. Treatment benefit-risk was assessed every 12 weeks.
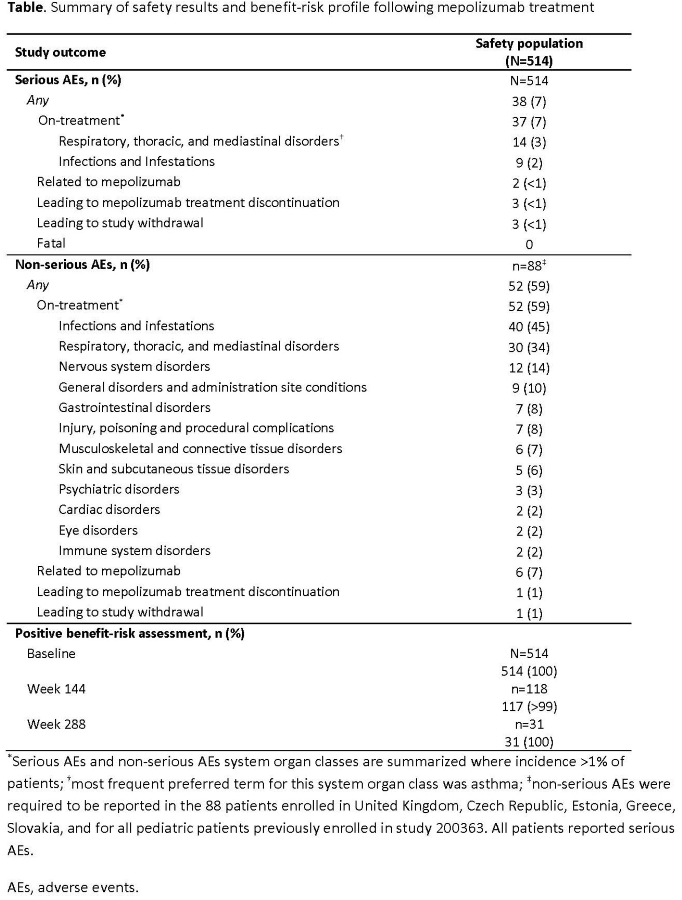
Results: Median (min,max) treatment duration (N=514) was 15.5 (1,77) months, equating to 919.8 patient years of study exposure. Safety results are reported in the Table. From 514 patients, 37 had on-treatment SAEs, 2 of which were deemed mepolizumab-related. Respiratory, thoracic, and mediastinal disorders were the most common SAEs. From 88 patients, 52 had on-treatment non-serious AEs, of which 6 were deemed mepolizumab-related. A positive benefit-risk profile was reported throughout the study.
Conclusions: The safety profile of mepolizumab in SEA aligns with previous reports; long-term treatment was well tolerated with a favorable benefit-risk profile.
Funding: GSK(201956,NCT00244686)