Abstract
Introduction: Exhaled nitric oxide (FeNO) is a marker for airway inflammation measured by portable or stationary analyzers. Usability of portable devices was not previously assessed and the influence of the participant's ease of analyzer use on FeNO results is unclear.
Methods: In a subset of the LEAD (Lung, hEart, sociAl, boDy) general population cohort, NIOX VERO (NV), NObreath (NB), Vivatmo pro (VP), and CLD88 analyzer (reference, REF) were compared with System Usability Scale, and Kruskal-Wallis tests for usability, and equivalence at a clinically relevant range of ?70 ppb with linear models and Bland-Altman plots.
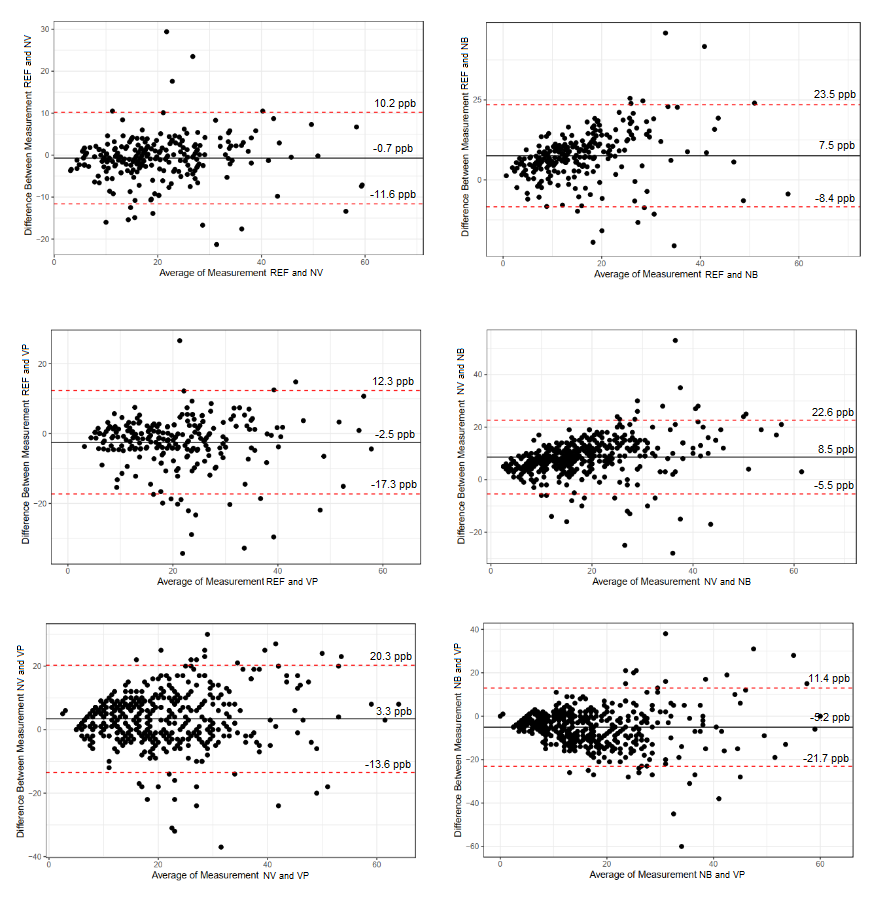
Results: In 486 participants (62±14 years old, 52% male, 10% current smokers, group of n=236 in usability study) FeNO values measured were clinically equivalent (Fig. 1) with a high overall agreement at essential thresholds (95% or above at ?40ppb).
All portable analyzers had a high usability score, with NB scoring best. Usability was not associated with inter-device differences (all p>0.05). VP required the least attempts and time to measurement success, followed by NB. All portable devices showed short learning curves, no critical problems, and good use in daily routine.
Conclusion: In this subset of the LEAD general population cohort, all portable devices had good usability and comparability, with the lowest difference to REF measured by NV, best usability score by NB, and shortest measuring time by VP.