Abstract
Introduction: On-treatment clinical remission (CR) is an aspirational goal in asthma management, with groups trying to agree on a definition. In a post hoc analysis of the real-world REDES study, after 52 weeks of mepolizumab, 37% of patients with severe asthma (SA) achieved a 3-component definition of CR (oral corticosteroid-free, exacerbation-free, Asthma Control Test score ?20).
Aim: Assess the impact of different lung function outcomes (as a fourth component) on CR outcome.
Methods: This post hoc analysis of REDES assessed the proportion of patients achieving a 4-component CR outcome (defined in Table) at Week 52.
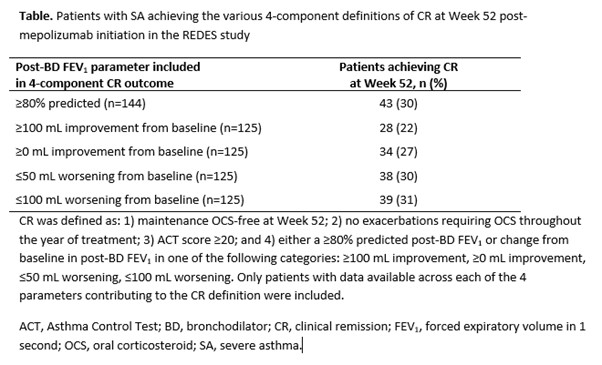
Results (Table): Including the ?80% predicted post-bronchodilator (BD) forced expiratory volume in 1 second (FEV1) parameter in the definition, reduced patients achieving CR to 30% vs the 3-component definition. Using the change from baseline in post-BD FEV1 categories, 22%?31% of patients achieved CR, increasing sequentially as the level of improvement in post-BD FEV1 required decreased.
Conclusions: These data confirm that mepolizumab is effective at achieving CR in SA. Similar outcomes were observed with the ?80% predicted FEV1 and ?50 mL worsening from baseline in post-BD FEV1 parameters, which may reflect natural loss of lung function that occurs with age. These findings provide insight into the definition of CR for SA, and the variation in outcome if lung function is incorporated.
Funding: GSK (213172)