Abstract
Aims
Bronchiectasis (BE) is a chronic inflammatory condition of the airways associated with chronic cough. The benefit of inhaled corticosteroids (ICS) and long-acting beta agonists (LABA) is unclear. We hypothesised that ICS/LABA could reduce cough symptoms in BE patients without asthma or COPD.
Methods
The FORZA study is a multicentre, randomised, double-blind, placebo-controlled trial of beclomethasone-formoterol aerosol 200/12 mcg twice-daily vs placebo in adult BE patients. Cough had to be present on most days for ?8 weeks. Patients with asthma or COPD, current smoking, and ICS use were excluded. Primary endpoint was change in cough at 3 months, using the Leicester Cough Questionnaire (LCQ). Secondary endpoints were change in QoL-B respiratory, mMRC, FEV1 and incidence of adverse events (AEs) including pulmonary exacerbations (PEs).
Results
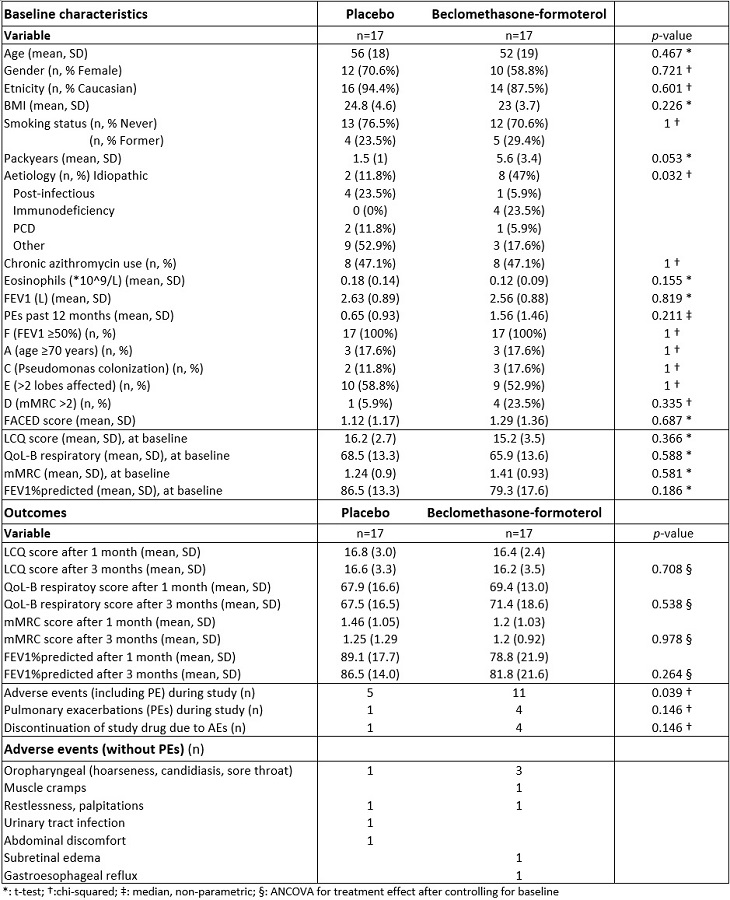
In total, 34 participants were included. The study was terminated early due to insufficient inclusions. After 3 months, both groups showed no difference in LCQ, QoL-B, mMRC, or FEV1. PEs occurred in 4/17 vs 1/17 participants and AEs (including PEs) in 11/17 vs 5/17 participants (p=0.039) of the beclomethasone-formoterol vs placebo group.
Conclusions
The use of ICS/LABA did not reduce cough in BE patients without asthma or COPD. However the incidence of significantly more adverse events warrants caution for prescribing ICS/LABA in this category of BE patients.