Abstract
Background
Neutrophilic inflammation and neutrophil serine proteases, e.g., neutrophil elastase (NE), play key roles in bronchiectasis (BE). Blood eosinophilia is present in ~20% of BE patients. The significance of eosinophilic inflammation in BE is unclear. Brensocatib, an investigational dipeptidyl peptidase-1 inhibitor, prolonged time to first exacerbation (Ex) and reduced sputum NE levels vs placebo in the phase 2 WILLOW study (NCT03218917).
Aim
To assess baseline characteristics and treatment outcomes by eosinophilic endotype (eosinophil count [EOS] ?300 cells/µl) among WILLOW patients.
Methods
Adults with BE treated with once-daily brensocatib (10 mg or 25 mg) or placebo were analysed by baseline blood EOS (<300 cells/µl or ?300 cells/µl). Endpoints were time to first Ex, annualised Ex rate, sputum NE level and treatment-emergent adverse events (TEAEs).
Results
Participants with baseline blood EOS ?300 cells/µl (49/255) had greater BE Severity Index scores and were more likely to receive inhaled steroids or maintenance macrolides, or have P. aeruginosa in sputum. Brensocatib prolonged time to first Ex, reduced annualised Ex rates and decreased sputum NE levels vs placebo in both subpopulations (table) 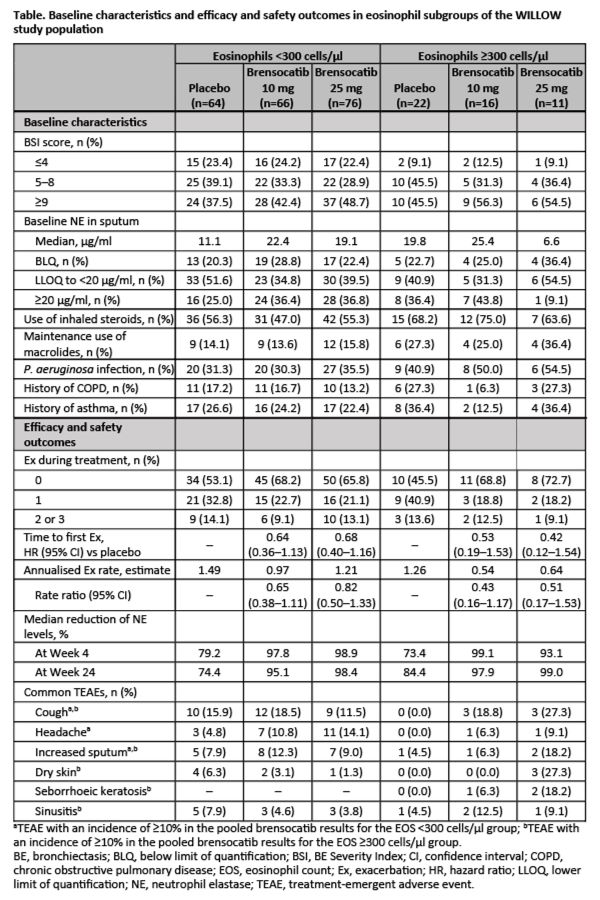
Brensocatib was well tolerated.
Conclusion
Brensocatib treatment in BE patients prolonged time to first Ex and reduced Ex rates and sputum NE levels vs placebo, regardless of eosinophilic subtype.