Abstract
Uncertainty exists about the safety and beneficial effects of delivering pulmonary rehabilitation (PR) during acute exacerbations of COPD (AECOPD). We explored the effects of a home-based PR programme during moderate AECOPD.
A randomized controlled trial was conducted (NCT03751670). Patients with AECOPD were randomly assigned to the control (CG, i.e., standard medication) or experimental (EG, i.e., standard medication plus 3-weeks of PR [breathing control, airway clearance, exercise, psychoeducational support]) group within 48h of the diagnosis (baseline). Symptoms (COPD assessment test, London chest activities of daily living, functional assessment of chronic illness therapy?fatigue), handgrip and quadriceps muscle strength, and functional capacity (short physical performance battery, 1-minute sit-to-stand test, Chester step test) were assessed at baseline and after 3 weeks. Comparisons within/between groups were explored with (non-)parametric mixed ANOVAs.
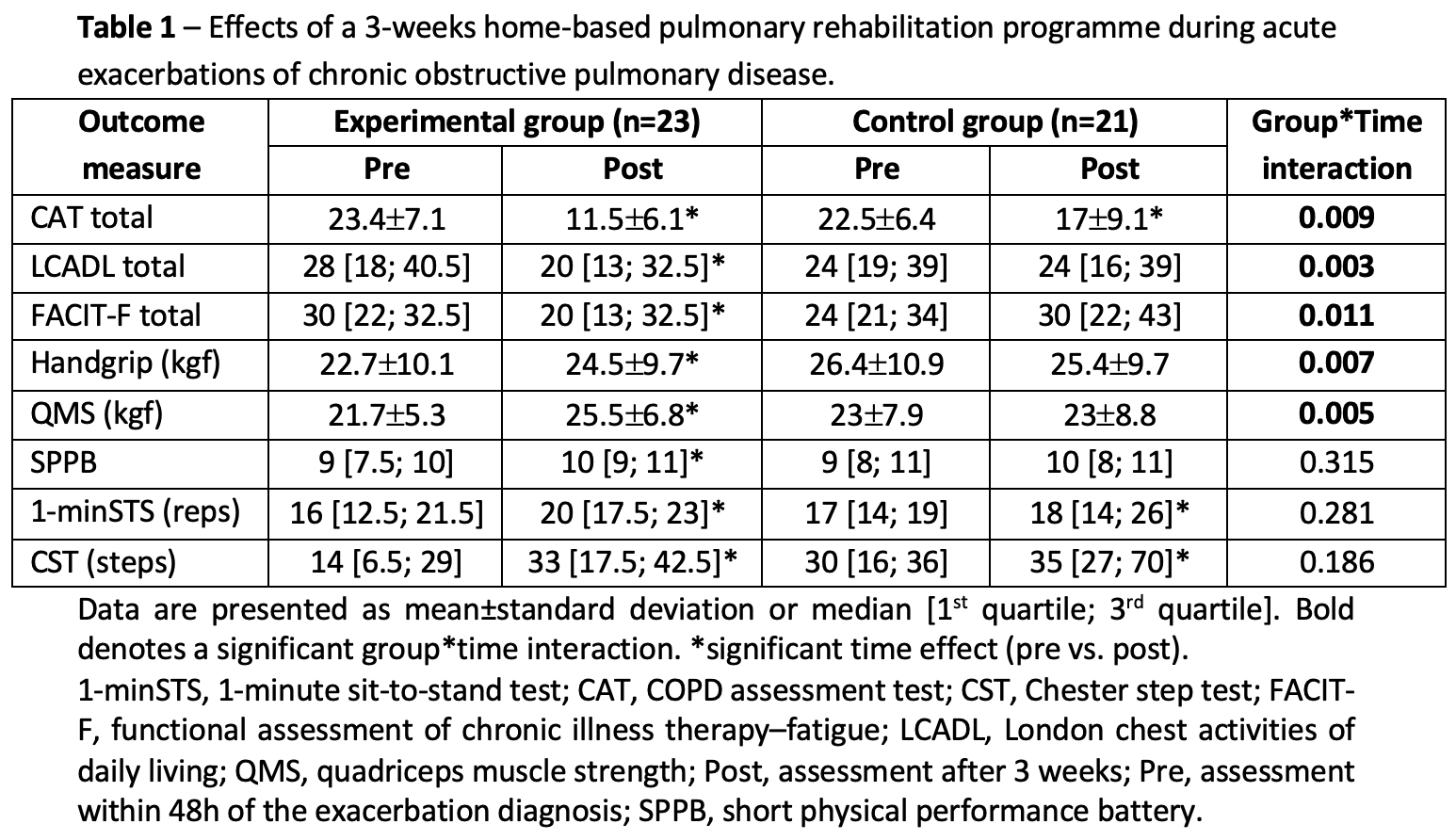
We included 44 patients (77% male, 68±10yrs; FEV1 48±18%pred). After 3 weeks, the EG presented significant improvements in all outcomes; symptoms and muscle strength improved significantly in the EG in comparison to the CG (Table 1). No adverse events were reported.
A 3-weeks home-based PR programme is safe and more effective than only standard medication in improving patients? symptoms and muscle strength during recovery of moderate AECOPD, outcomes often associated with poor prognosis.