Abstract
Introduction: Blood-based biomarkers predictive of progression of pulmonary fibrosis would be of clinical value.
Aim: To investigate associations between circulating biomarker levels at baseline and outcomes in subjects with progressive fibrosing interstitial lung diseases other than idiopathic pulmonary fibrosis (IPF) in the INBUILD trial.
Methods: Associations between baseline biomarker levels and i) rate of decline in FVC (mL/year) over 52 weeks, ii) time to ILD progression (absolute decline in FVC ?10% predicted) or death over 52 weeks, iii) time to death over the whole trial were assessed in subjects who received placebo.
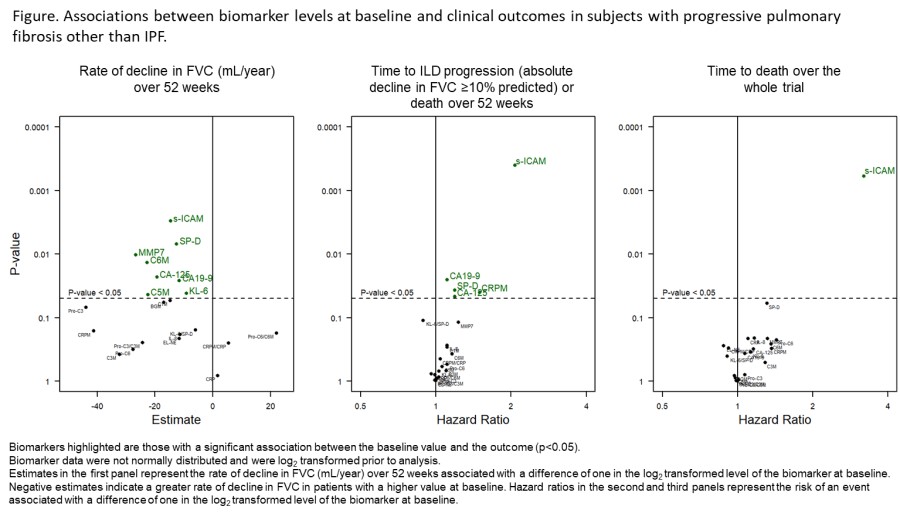
Results: In total, 331 subjects received placebo. Median exposure to placebo was 17.4 months. Higher baseline levels of s-ICAM (a marker of inflammation), KL-6, SP-D, CA-125 and CA19-9 (markers of epithelial injury), and C5M, C6M and MMP-7 (markers of ECM turnover) were associated with a greater rate of decline in FVC over 52 weeks (Figure). Higher baseline levels of s-ICAM, SP-D, CA-125, CA19-9 and CRPM were associated with an increased risk of ILD progression or death over 52 weeks. Higher baseline levels of s-ICAM were associated with an increased risk of death over the whole trial.
Conclusions: Data from the INBUILD trial identified circulating biomarkers that may be associated with worse outcomes in subjects with progressive pulmonary fibrosis. Further studies in other cohorts are needed.