Abstract
Introduction: Previous studies have suggested that higher neutrophil count and lower lymphocyte count are associated with lung function decline and mortality in subjects with idiopathic pulmonary fibrosis (IPF).
Aim: To investigate associations between blood neutrophil and lymphocyte counts at baseline and outcomes in the INBUILD trial in subjects with progressive fibrosing interstitial lung diseases (ILDs) other than IPF.
Methods: We assessed associations between neutrophil count ?Q3 vs >Q3 and lymphocyte count ?Q1 vs >Q1 at baseline and time to i) death, ii) acute exacerbation of ILD or death, and iii) ILD progression (absolute decline in FVC ?10% predicted) or death.
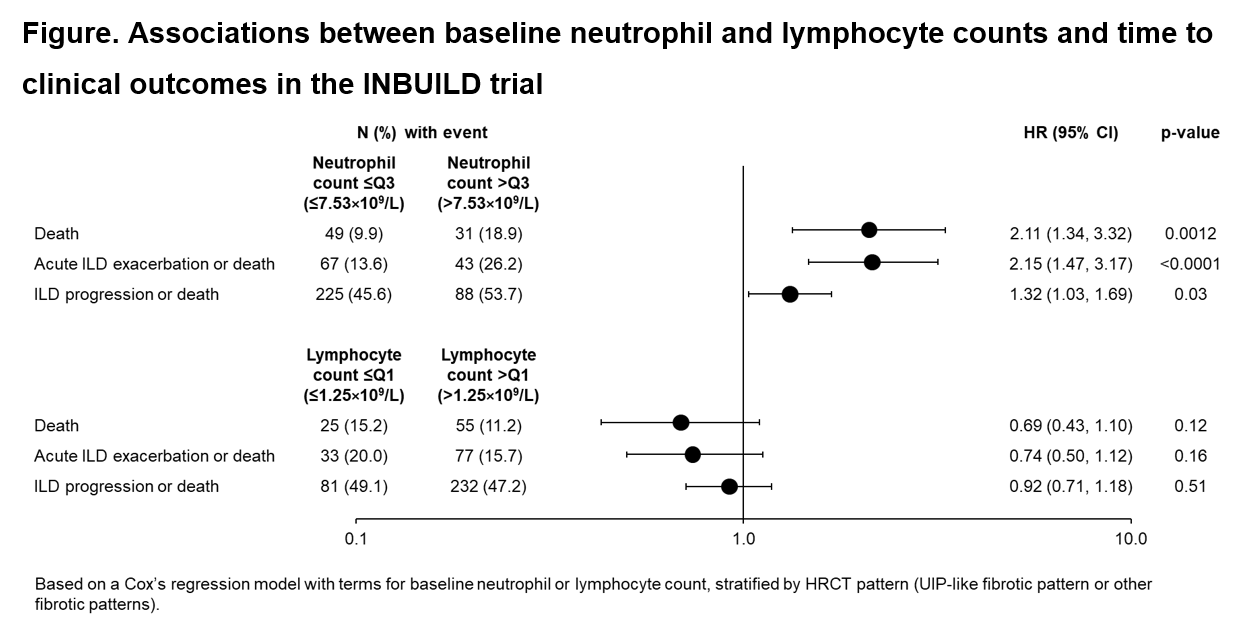
Results: A total of 663 subjects received trial medication (nintedanib or placebo) for a median of 17.4 months. At baseline, median (Q2) neutrophil count was 5.67×109/L and Q3 was 7.53×109/L, while median lymphocyte count was 1.75×109/L and Q1 was 1.25×109/L. The risks of death, acute exacerbation of ILD or death, and ILD progression or death were significantly greater in subjects with neutrophil count >Q3 vs ?Q3 at baseline (Figure). A non-significant reduction in the risks of these outcomes was observed in subjects with lymphocyte count >Q1 vs ?Q1 at baseline.
Conclusions: Data from the INBUILD trial suggest that higher neutrophil count may be associated with worse outcomes in subjects with progressive pulmonary fibrosis.