Abstract
Background/Aim: Acid sphingomyelinase deficiency (ASMD), a multisystemic, progressive, debilitating lysosomal storage disease, has prominent pulmonary manifestations due to sphingomyelin accumulation in alveolar, bronchial, and pleural macrophages. Diagnostic delays are common due to low disease awareness. We evaluated the effect of olipudase alfa, intravenous recombinant human ASM (Sanofi), recently approved in the EU and US for the non-CNS manifestations of ASMD, on respiratory outcomes.
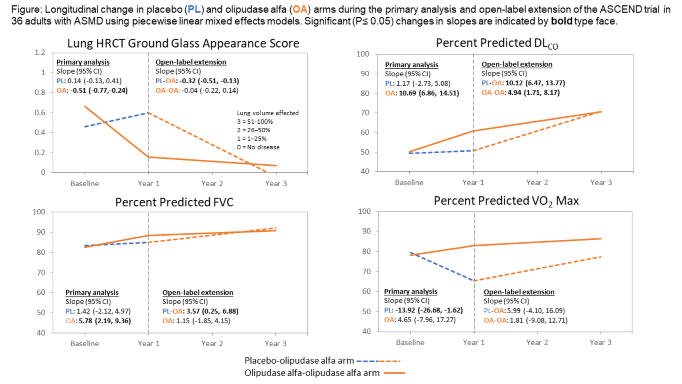
Method: Piecewise linear mixed effects models were used to compare change in pulmonary function tests and maximal exercise capacity between the 1-year primary analysis of the placebo-controlled ASCEND trial (NCT02004691, N=36 adults with ASMD) and after up to 2 years in the open-label extension.
Results: Olipudase-alfa, but not placebo-treated patients, had improved radiological infiltrates (clearance of ground glass opacities), lung function (increased FVC), diffusion (increased DLCO), and exercise capacity (O2 uptake) (figure). Olipudase alfa was well tolerated; no patients have withdrawn from the trial due to drug-related adverse events.
Conclusion: Olipudase alfa enzyme replacement therapy addresses the underlying metabolic defect in ASMD. Sustained and progressive improvement was seen in pulmonary imaging, gas exchange, lung mechanics, and exercise testing.