Abstract
Post tuberculosis lung disease has largely been characterized using spirometry. Data on complete pulmonary function including diffusion capacity for carbon monoxide (DLCO) and total lung capacity are lacking.
The ongoing StatinTB trial (NCT04147286), evaluates safety/efficacy of 40 mg atorvastatin to reduce persistent lung inflammation (PLI) after TB treatment in HIV-/HIV+ adults measured by 18F-FDG-PET/CT. Follow-up is 96 weeks. We report complete pulmonary function test with DLCO and nitrogen washout for functional residual capacity (FRC), using EasyOne Pro®Lab (ndd, Zürich, Switzerland), for 106 participants at enrolment into StatinTB/ExtendTB. The trial is conducted according to ICH-GCP.
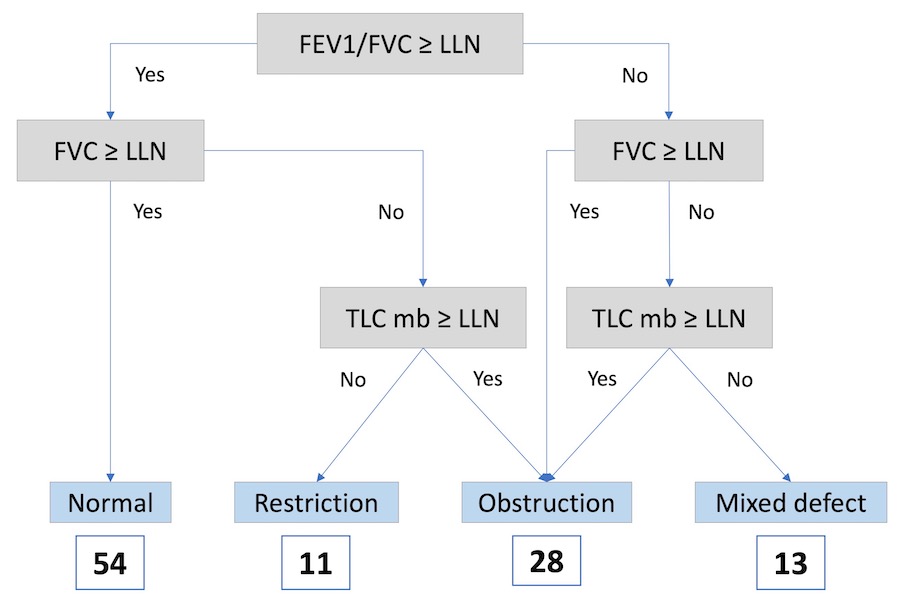
106 participants (32.1% women) aged 34.8±11.3 years were enrolled; 20.8% were HIV+, 57.5% smokers, and 28.3% had previous TB. 50.9% of participants had normal lung function by lower limit of normal (LLN) standard, 10.4% had restriction, 26.4% obstruction, and 12.3% a mixed defect (Figure 1). Normal DLCO%Pred (>75%) was measured in 62.3% of participants; 24.5% had mild, 11.3% moderate and 1.9% severe impairment of diffusion capacity (DLCO%Pred: 60-75%, 40-60%, <40%).
Only half of patients post TB treatment have normal lung function based on lung volumes and 37.7% suffer from diffusion impairment. Further studies are urgently needed to assess if those patients benefit from specific therapies against post TB lung disease.