Abstract
Introduction
Phosphodiesterase-5 inhibitors (PDE5is), primarily sildenafil, are often used off-label in patients with pulmonary hypertension due to ILD (PH-ILD), despite a lack of controlled clinical trial data supporting their use. The safety of PDE5is with inhaled treprostinil (iTre) has not been studied.
Aims and objectives
To evaluate the safety of adding PDE5is in patients receiving iTre for PH-ILD in the INCREASE open-label extension (OLE).
Methods
The INCREASE OLE enrolled patients from the INCREASE randomized controlled trial (RCT) and allowed the addition of PDE5is, which were not permitted in the INCREASE RCT. All OLE patients received iTre. Endpoints of interest were SpO2, supplemental O2, exacerbations, hospitalizations, and death.
Results
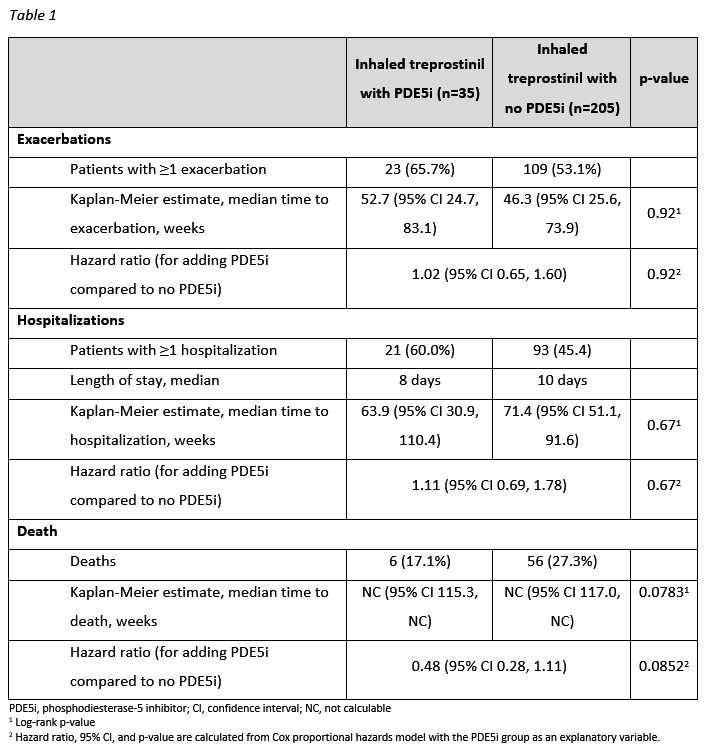
35 patients added a PDE5i during the OLE (32 sildenafil, 3 tadalafil). Patients who added a PDE5i had more severe baseline hemodynamics. Median iTre exposure in the PDE5i and no PDE5i groups were 95.0 and 70.4 weeks, respectively. SpO2 changes during the 6MWT were similar between groups at Week 48 and 96. Supplemental O2 at rest and during the 6MWT were similar. The PDE5i group did not have increased risk of exacerbations, hospitalizations, or death.
Conclusions
In this small cohort of PH-ILD patients with on inhaled treprostinil, PDE5is were added without worsening oxygenation or increasing risk of clinical events.