Abstract
Introduction
Long term symptoms post-COVID contribute significantly to morbidity of COVID infections. Intranasal Chlorpheniramine Maleate (iCPM) has shown anti-inflammatory and COVID-19/respiratory virus anti-viral effects.
Aims and objectives
This study aimed to assess the difference in the development of post-COVID symptoms between participants who received a 10 day treatment of iCPM and placebo at a safety check after SARS-CoV-2 infection.
Methods
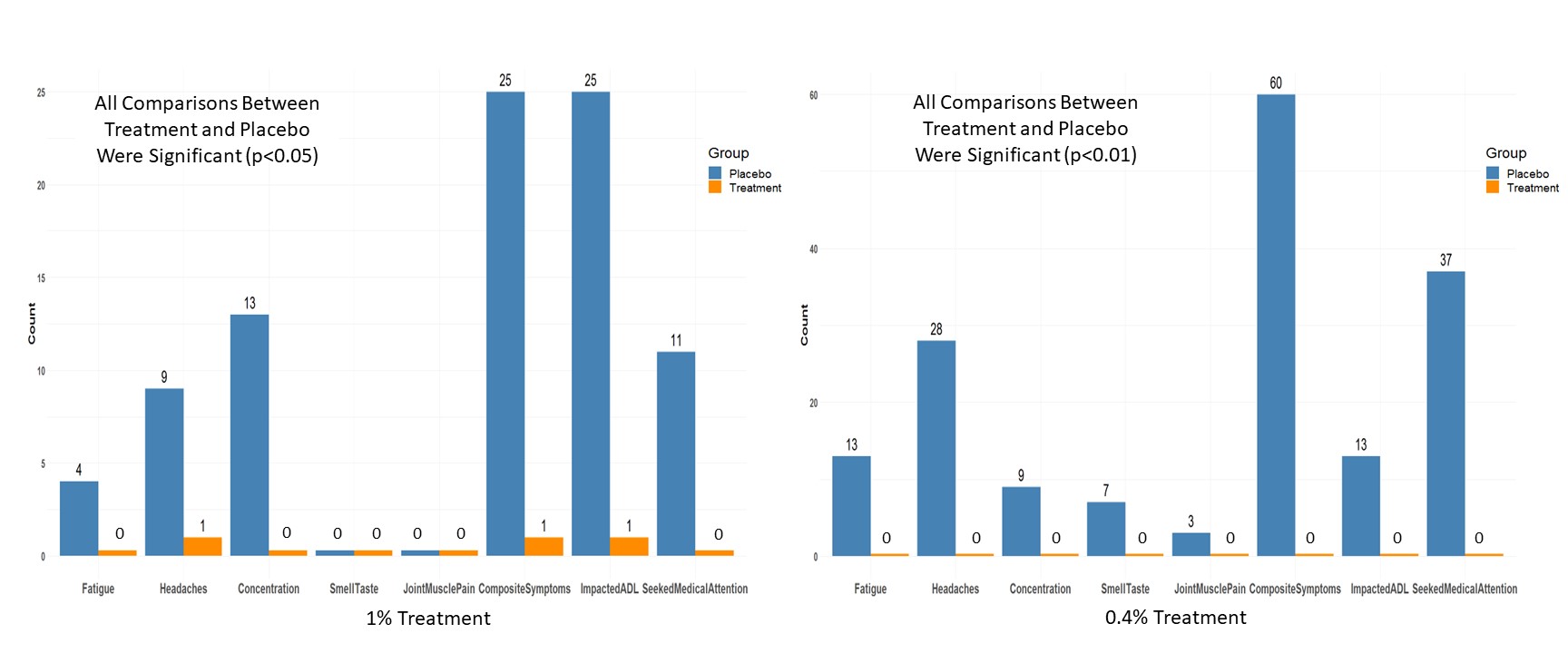
Safety phone call checks, involving a 17-question survey of symptoms were conducted on COVID-19 clinical trial participants of two trials of iCPM vs. placebo. Study I and II were performed with doses 1% and 0.4%, with prevalent variants being 23A and 22F of Omicron respectively. These checks occurred 5-16 months in Study I and and 3-9 months for Study II after participation.
Results
Safety calls in Studies I and II enrolled 46 & 74 subjects in placebo and 56 & 83 in control respectively. Most symptoms were absent, but significant differences were observed between placebo and control if the answer was positive (Figure 1).
Conclusions
The findings of safety check of two randomized controlled trials indicate that participants who received the iCPM had significantly less long term post-COVID symptoms. This suggests a potential benefit from iCPM in preventing or reducing the occurrence of these symptoms.