Abstract
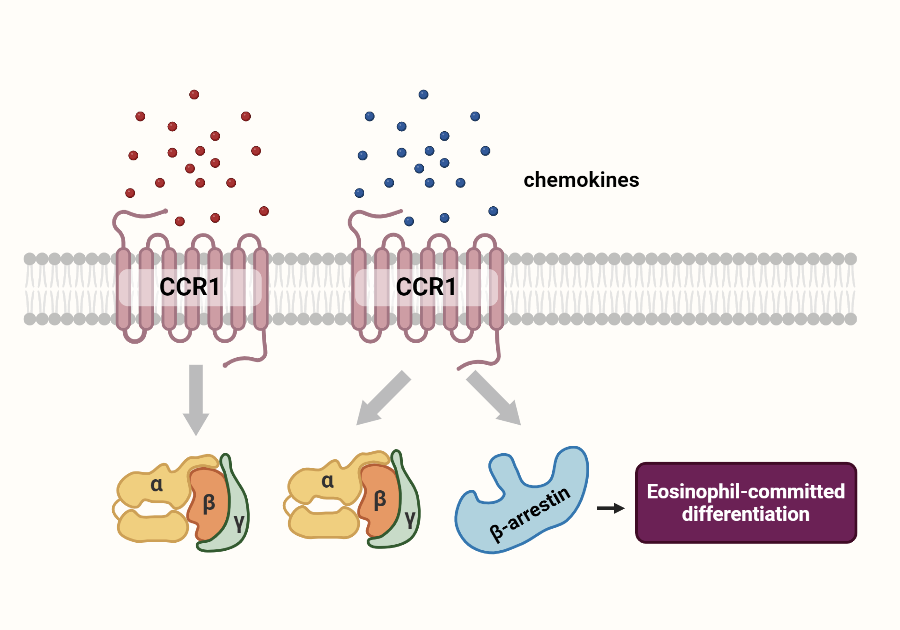
Chemokine receptor CCR1 mediated the eosinophil-committed differentiation, regarded as a potential therapeutic target for asthma treatment. CCR1 belonged to G protein-coupled receptors (GPCRs). Our previous studies revealed that CCR1 preferentially activated either G protein or ?-arrestin-dependent signaling upon different ligands binding, which was called biased signaling. At many GPCRs, signaling events mediated by G protein and ?-arrestin were shown to have distinct physiological actions from one another. An accurate evaluation of biased signaling was crucial for preclinical drug discovery. But the function of CCR1 biased signaling in asthma remained unclear.
According to the results of single-cell sequencing, our preliminary studies indicated increased expression of ?-arrestin-related proteins in the asthmatic mice. Blockade of ?-arrestin signaling impaired eosinophil-committed differentiation. These results suggested that CCR1 induced eosinophil-directed differentiation via biased ?-arrestin activation, leading to the development of asthma.
BX471 is one of the clinical candidates targeted CCR1. Intriguingly, we found that BX471 selectively inhibited G protein, rather than ?-arrestin signaling of CCR1, which might contribute to its failure in clinic trials. Thus, our studies identified the key function of CCR1 biased signaling in asthma pathogenesis, as well as providing the basis for the design of CCR1-targeted biased antagonists for asthma treatment.