Abstract
Introduction: In individual placebo-controlled trials in subjects with idiopathic pulmonary fibrosis (IPF) and other forms of progressive pulmonary fibrosis (PPF), treatment with nintedanib was associated with numerical reductions in mortality.
Aim: To assess the effect of nintedanib on the risk of mortality across trials in subjects with IPF and PPF.
Methods: Meta-analyses of the effect of nintedanib versus placebo on time to death, and time to first acute exacerbation of ILD or death, were performed using data from randomised placebo-controlled trials in subjects with IPF (TOMORROW, INPULSIS-1 and -2, a Phase IIIb trial [NCT01979952]) and other forms of PPF (INBUILD). The heterogeneity of the effect of nintedanib across trials was assessed using the ?2 statistic, ?2 and Q test p-value.
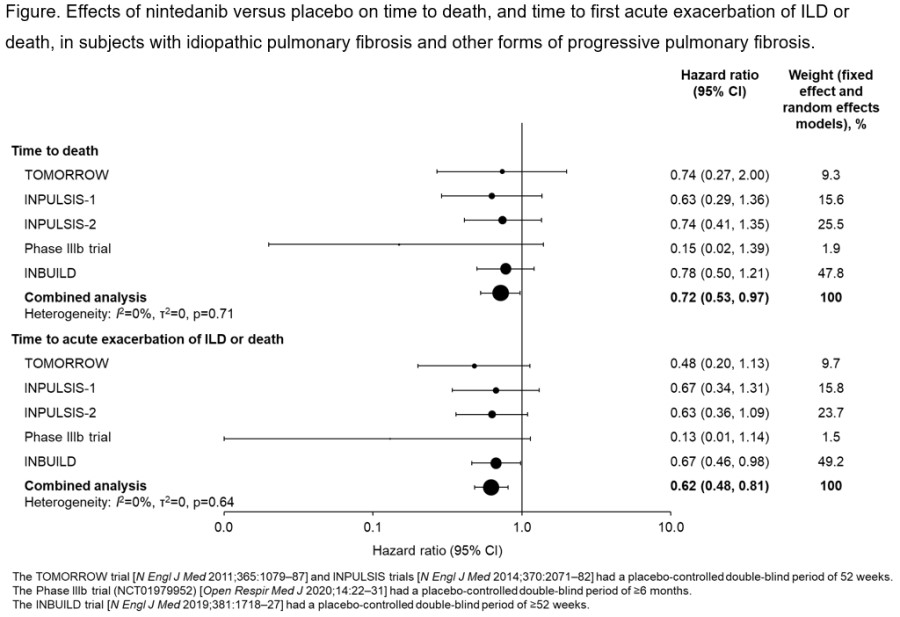
Results: In the combined analysis of data from 5 clinical trials involving 2007 patients, nintedanib reduced the risk of death (HR 0.72 [95% CI: 0.53, 0.97]) and the risk of acute exacerbation of ILD or death (HR 0.62 [95% CI: 0.48, 0.81]) compared with placebo. The results were consistent across the trials (Figure).
Conclusions: Nintedanib had consistent effects on reducing the risk of mortality, and of acute exacerbation of ILD or mortality, across clinical trials conducted in subjects with IPF/PPF.