Abstract
Introduction
High discontinuation rates due to adverse effects of nintedanib are well documented, however, there are little data examining the duration of treatment prior to discontinuation.
Aims and objectives
In this single-centre study, we performed a time-to-event analysis of adverse effects and discontinuation of nintedanib in Idiopathic Pulmonary Fibrosis (IPF) patients.
Methods
All patients initiated on nintedanib over a 5-year period were followed up for the duration of therapy by the IPF nurse specialist team. Baseline demographic data, date of nintedanib initiation, recording of first advent event, and medication discontinuation were recorded.
Results
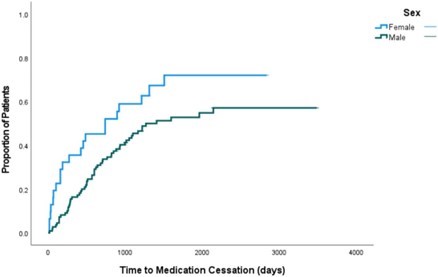
147 patients, mean age 70.5 (SD=6.7), 79% men (n=116) were included in this study. 80 (54%) of patients stopped taking nintedanib due adverse effects. The median time-to-medication discontinuation was shorter in women than in men (270 days vs 567 days, p=0.049). Women were significantly more likely than men to stop taking nintedanib as seen in figure 1 (HR=2.33 [95% CI = 1.25-4.35], p=0.007). Older age was also a significant predictor of medication discontinuation (HR=1.87 [95% CI = 1.04-3.35], p=0.035).
Conclusions
Our time-to-event analysis indicates gender inequality in long term tolerability of nintedanib, which may be explained by gender difference in the pharmacokinetics of nintedanib. Future clinical trials should strive to understand how therapeutics may affect men and women differently.