Abstract
The AIR trial (NCT04533022) is a multi-center, single-arm trial evaluating safety and efficacy of the angiotensin II type 2 receptor agonist (ATRAG) C21 in patients with Idiopathic Pulmonary Fibrosis (IPF).
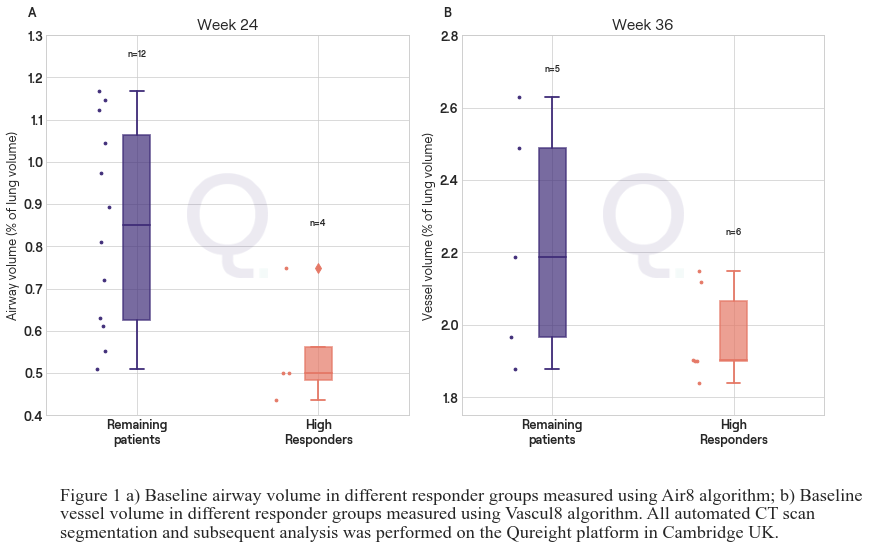
Interim results identified a group of patients with improved lung function, defined as increase from baseline in FVCpp ?10% per annum, following 24 weeks C21 treatment. Classifying these patients as ?high responders?, automated segmentation of baseline CT scans was performed to quantify volumes of lungs, airways, blood vessels and fibrotic tissue. Results showed that the high responder group had lower airway volumes at baseline vs remaining patients (p=0.02) (Fig 1a). There is also a trend in 36-week data indicating that high responders may have lower vessel (1b, mean 2.0% sd 0.13% vs. 2.2% sd 0.3%) and fibrosis (mean 13% sd 6% vs. 20% sd 11%) volumes at baseline vs the remaining patients, but these differences were not statistically significant.
We have previously shown that automated CT-based airway volume measurements correlates with IPF disease severity1. Although only a smaller number of patients have completed 24 weeks and had baseline imaging available, these results suggest that patients with early IPF, and thus more limited disease, may respond better to C21. This is in keeping with C21?s reparatory mechanism of action targeting biologically active tissue.
1. Roberts et al ERJ 2021