Abstract
Introduction: The open-label ATLAS study in IPF demonstrated a statistically significant benefit of AP01 100mg BID compared to 50mg OD, based on the slopes of mean change in FVC% predicted over 48 weeks (?0.4% [95% CI: ?3.2, 2.3]) vs ?4.9% [?7.5, ?2.3], respectively. The difference in slopes (100mg BID ? 50mg OD) was 4.5 (95% CI: 0.7, 8.2; p=0.022) at 48 weeks (West et al, Thorax, 2023).
Aim: To compare the open-label ATLAS data with data from 3 published IPF RCTs to assess AP01 efficacy relative to placebo.
Methods: The ATLAS population was matched to each RCT on key baseline characteristics by assigning weights to ATLAS subjects (Signorovitch JE, et al. Value Health 2012;15:940-7). A random slopes model was used to estimate the mean annual rate of FVC decline in the AP01 100mg BID arm; estimates were compared by Z-test to the published RCT placebo rates.
Results: AP01 100mg BID demonstrated a statistically significant (p<0.05) benefit compared to placebo in all 3 RCTs (Figure 1).
Figure 1 Difference in FVC Decline (mL) in ATLAS (randomized n=45) Compared to Placebo in IPF RCTs
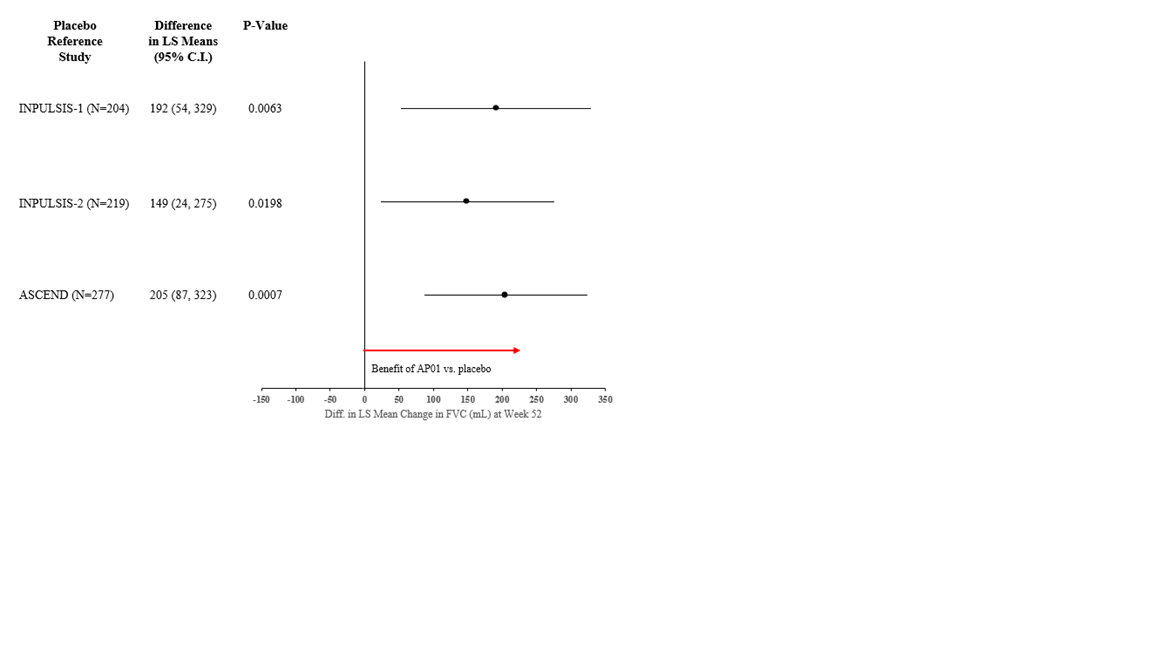
Conclusion: The statistically significant differences seen when comparing AP01 100 mg BID to placebo in 3 RCTs support the efficacy findings from the ATLAS study and progression of this AP01 dose into Phase 2b/3 interstitial lung disease studies.