Abstract
INNA-051 is a TLR2/6 agonist in development as an innate immune modulator, delivered using a nasal spray to target the primary entry site of viral respiratory infections. A randomised, placebo-controlled influenza human challenge study was conducted in 123 healthy adults to evaluate the effect of INNA-051 on the course of viral infection and host response. Laboratory-confirmed infected participants with a Heamagglutination Inhibition titre of 10 against A/Perth/16/2009 (H3N2) virus, at time of challenge, were included in the analysis.
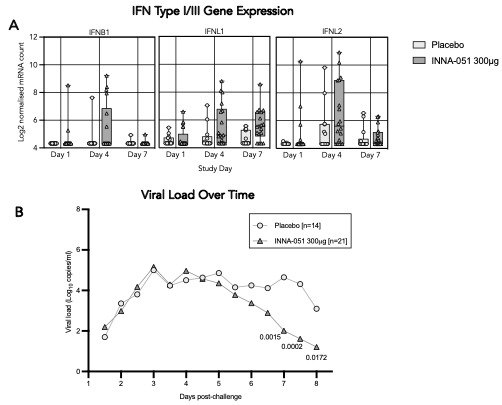
Host responses to influenza infection, including interferon type I and III gene expression were analyzed using the Nanostring Counter Human Pan Cancer Immune Profiling panel from nasal swabs of INNA-051 treated and placebo treated PCR-confirmed infected participants at D1, 4 and 7 post-viral challenge.
INNA-051 was found to increase host responses against influenza and interferon type I & III gene expression compared to placebo treatment, reflecting accelerated viral clearance observed in this study (Figure 1 A/B, respectively).
With the safety profile observed in this study and previously in a Phase 1 study, these results support further clinical development of INNA-051 in the context of natural viral respiratory tract infections in individuals at increased risk of severe illness.