Abstract
Introduction
Inhaled triple therapy (TT) is recommended for patients with asthma with poor symptom control and to reduce risk of exacerbations. We assessed patient characteristics of those who initiated SITT with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) following treatment with dual therapy inhaled corticosteroids and long-acting ?2?agonist (ICS/LABA) in routine practice.
Methods
A longitudinal cohort of patients with asthma was identified in a US Optum administrative claims database. Patients ?18 years with two years of continuous enrollment who initiated TT during Sep 2020?Mar 2021 following treatment with an ICS/LABA were included. Patients were stratified by initial dose of FF/UMEC/VI (TT100 or TT200). Baseline resource use was reported as annual rates and proportions.
Results
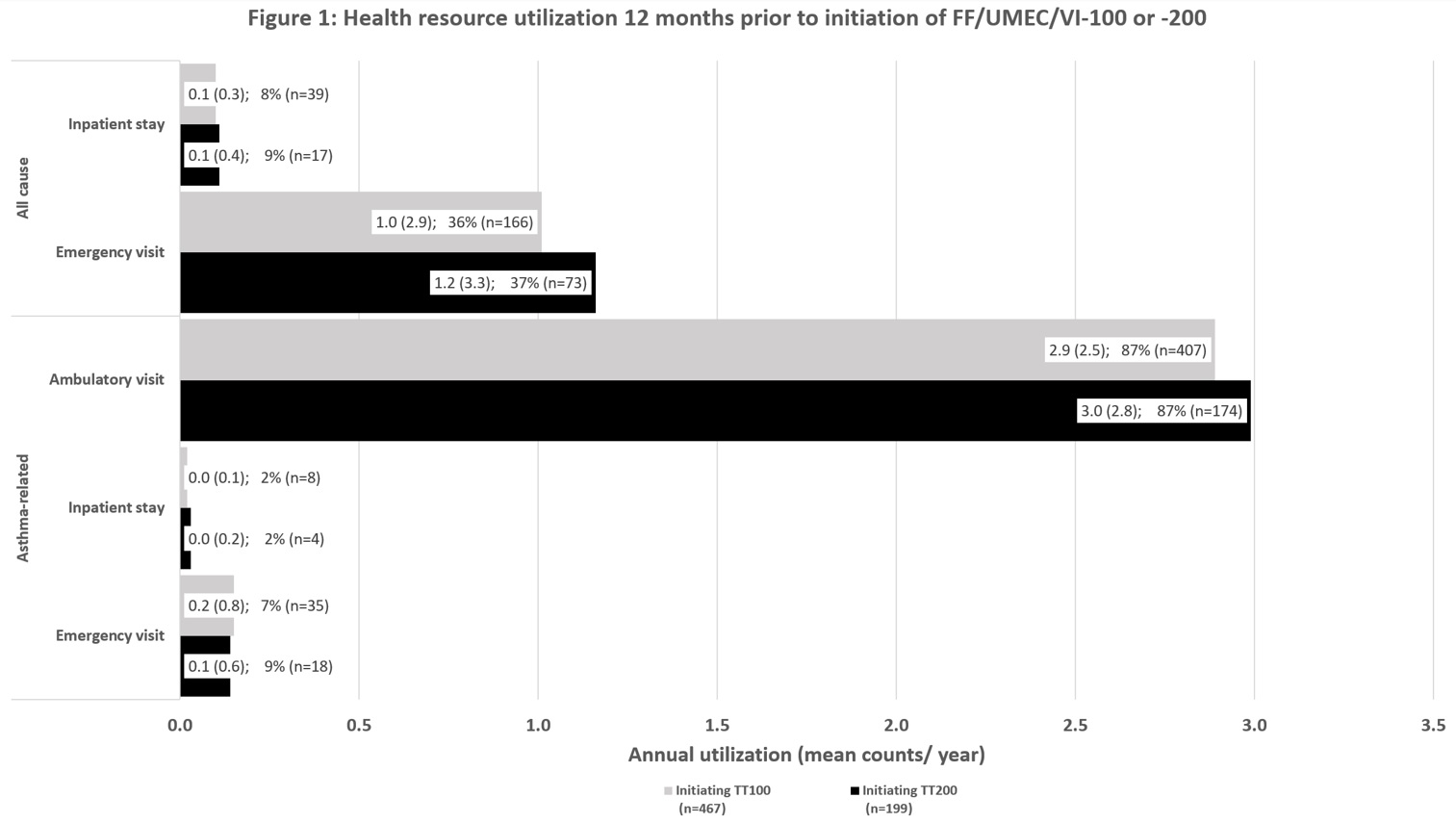
This study included 666 patients. Mean age was 57 years (65% female; mean comorbidity score=1.6) for TT100 and 62 (69% female; mean comorbidity score=1.8) for TT200. More patients who initiated TT200 were treated with a LAMA monotherapy (7 vs 3%) and oral corticosteroids (61 vs 57%) than TT100, prior to FF/UMEC/VI. All-cause and asthma-specific resource use was similar across both groups before TT initiation (Figure 1).
Conclusions
Differences in baseline clinical characteristics were noted between TT100/200 groups, with older and more severe patients initiating high dose FF/UMEC/VI.
Funding: GSK (217417)