Abstract
Background: Biologic therapies have made clinical remission a viable goal in patients (pts) with severe eosinophilic asthma (SEA).
Aim: We describe clinical remission in pts with SEA, with/without prior biologic experience, over 2 years of benralizumab in the large-scale, real-world, XALOC-1 study programme.
Methods: This analysis of retrospective, multinational data assessed clinical remission rates over 2 years. Remission was defined as: exacerbation free during the entire follow-up period (48 or 96 weeks [wks]); asthma symptom control (Asthma Control Test score ?16 or 6-item Asthma Control Questionnaire score <1.5) and no maintenance oral corticosteroid use, assessed at Wk 48 or 96.
Results: Pts (n=1070) had a mean (SD) age of 55.2 (13.7) years; 58.7% were female; 61.9% were biologic-naïve and 37.8% -experienced (omalizumab 43.6%, mepolizumab, 62.6%, reslizumab, 8.2%). 45.1% of pts achieved remission by Wk 48, 37.5% by Wk 96 (Figure). Of pts who met remission criteria at Wk 96, 59.3% had sustained remission from Wk 48. Remission rates were higher in biologic-naïve vs -experienced pts, and in omalizumab- vs mepolizumab-experienced pts, at all timepoints (Figure).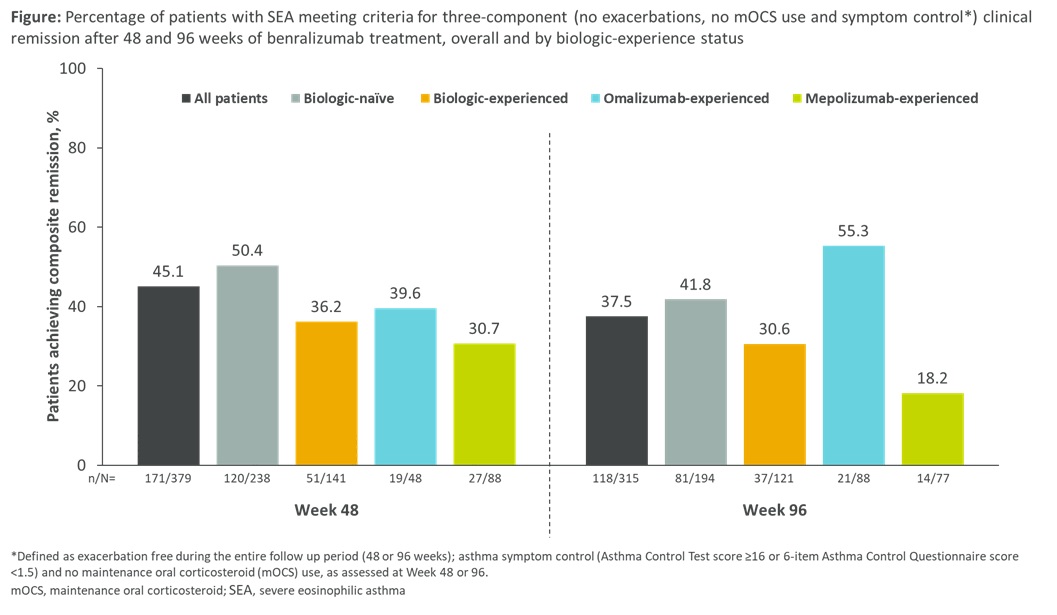
Conclusion: Clinical remission is a realistic, sustainable goal up to 2 years for pts with SEA receiving benralizumab, regardless of prior biologic experience.
Funding: AstraZeneca