Abstract
Background: Benralizumab is effective as add-on therapy in patients (pts) with uncontrolled severe eosinophilic asthma (SEA), a prevalent disease in Asia.
Aim: To examine the efficacy and safety of benralizumab in pts from Asia with SEA.
Methods: MIRACLE (NCT03186209) was a randomised, double-blind Phase 3 study in China (including Taiwan), South Korea and Philippines that stratified pts aged 12ญญ?75 with uncontrolled SEA on medium- to high-dose ICS-LABA 2:1 by baseline blood eosinophil count (bEOS; ?300 or <300/?L). Primary endpoint was annual asthma exacerbation rate (AAER) over 48 wks in pts with bEOS ?300/?L. Pre-bronchodilator (BD) FEV1 and total asthma symptom score (TASS) at Wk 48 were key secondary endpoints. Safety was evaluated by adverse events (AEs).
Results: 695 pts were randomised; 236 benralizumab 30mg and 237 placebo (PBO) had bEOS ?300/?L. Significant improvements in AAER of 74% (rate ratio 0.26 [95% CI 0.19, 0.36], p<0.0001), and differences in pre-BD FEV1 (0.25 L [95% CI 0.17, 0.34], p<0.0001) and TASS (?0.25 [95% CI ?0.45, ?0.05], p=0.0126) were seen for benralizumab vs PBO in pts with bEOS ?300/?L (Figure). AEs were similar with benralizumab (76%) and PBO (80%).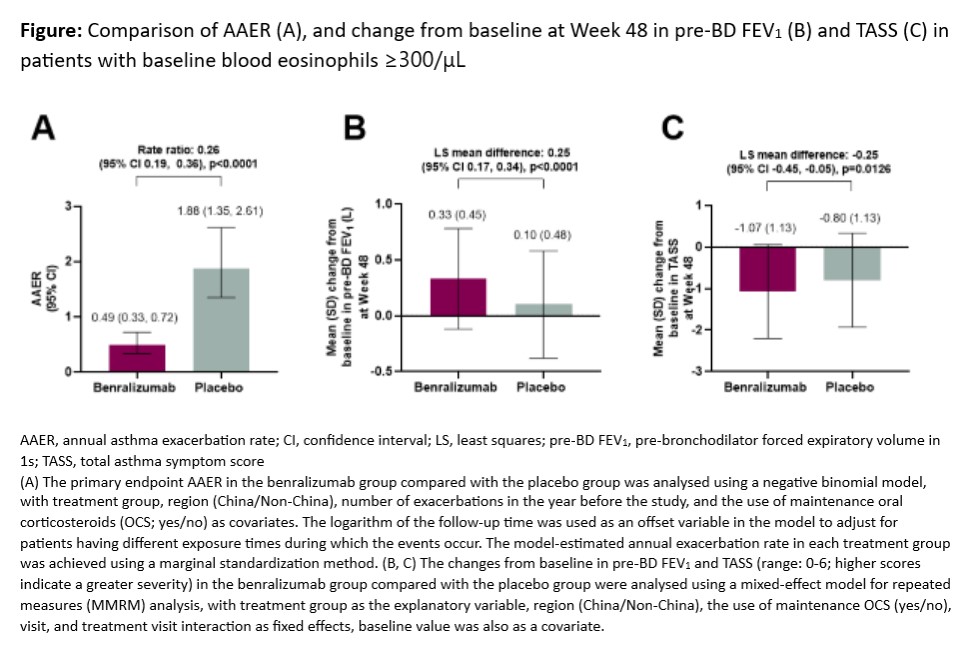
Conclusions: These data reinforce the efficacy and safety of benralizumab for SEA; results in an Asian population were consistent with the known global profile