Abstract
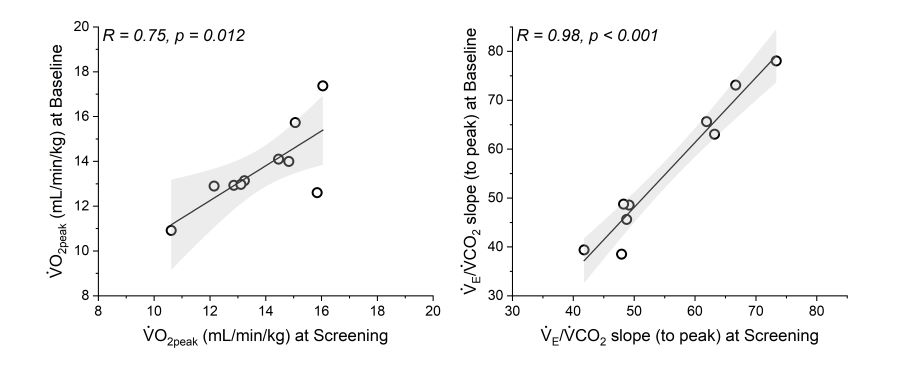
ADVANCE CAPACITY is a 28-week global, multicenter, randomized, double-blind, placebo-controlled study using VO2peak from a cardiopulmonary exercise test (CPET) to evaluate the efficacy of ralinepag (a selective, oral non-prostanoid, IP receptor agonist) to increase exercise capacity in subjects with pulmonary arterial hypertension (PAH). Aim: To evaluate the reproducibility of CPET variables in subjects with PAH when rigorous training and quality control processes are implemented. Methods: Following extensive training to qualify CPET laboratories, subjects (n = 10) performed a 10W/min ramp incremental CPET to intolerance during a screening (SCR) and a baseline (BSL) visit (performed within 4 weeks of one another). CPET data were processed by a core laboratory. Results: Subjects (1 male/9 female) were 54±16 years old, predominantly white and 4.2±4.0 years since PAH diagnosis. Over 80% had idiopathic PAH or PAH associated with connective tissue disease. Most subjects (90%) were on dual PAH background therapies and 78% were functional class II. Mean VO2peak at SCR was 13.8±1.7 mL/min/kg and at BSL was 13.7±1.8 mL/min/kg with the coefficient of variation (CV) 6.4%. The mean VE/VCO2 slope (to peak) was 55.2±10.3 at SCR and 54.9±14.0 at BSL, with the CV 5.8%. Conclusion: CPET variables are highly reproducible in PAH patients and are robust endpoints to assess exercise capacity in PAH.